The disadvantages of the long-term administration of antiretroviral therapy as well as the huge number of affected persons have placed the cure of HIV as a primary goal of Public Health. HIV may persist in the organism by at least four mechanisms: a latently infected cellular reservoir, the persistent replication of HIV in spite of antiretroviral therapy (ART), anatomic sanctuaries, and the immune dysfunction. Several strategies directed against these mechanisms have been developed. With all this, a complete eradication of HIV has been achieved in a patient using the transplantation of haemopoietic stem cells that were resistant to HIV-infection, and there are examples of functional cure either spontaneously (elite controllers) or after antiretroviral therapy (post-treatment controllers). However, no strategies have been successful in reducing the reservoir size, nor in achieving constant, uniform remissions. The failure of isolated strategies makes it likely that the combination of several of them may be the future solution.
Los inconvenientes de la administración indefinida del tratamiento antirretroviral y el enorme número de personas infectadas convierten la cura del VIH en un objetivo primordial de salud pública. El VIH puede persistir en el organismo por al menos cuatro mecanismos: un reservorio celular latentemente infectado, la replicación persistente a pesar del tratamiento, los santuarios anatómicos y la disfunción inmunológica. Se han desarrollado estrategias para erradicar el VIH haciendo frente a estos mecanismos. Hasta el momento, se ha conseguido la erradicación completa del virus en un paciente mediante el trasplante de progenitores hematopoyéticos resistentes a la infección y existen casos de curación funcional de modo natural (controladores de élite) o tras tratamiento antirretroviral (controladores postratamiento). Sin embargo, ninguna estrategia ha conseguido disminuir el reservorio, ni lograr remisiones de modo constante y uniforme. El fracaso de las estrategias aisladas sugiere que la combinación de varias de ellas sea la solución futura.
It has been rightly said that antiretroviral therapy (ART) is one of the most important advances in modern medicine. Since the introduction of ART, the combining of antiretroviral drugs has prevented the death and suffering of millions of people all over the world and brought a halt to the logarithmic expansion of the epidemic. Moreover, through access to generic drugs, the benefits of ART have been extended to countries with limited resources, where the need is greatest.
Nevertheless, ART does not completely eliminate HIV from the infected organism. Almost universally, when ART is stopped, the virus reappears in the blood within a few days or weeks. This means that the infected person is forced to continue taking ART throughout their entire life, and that too is associated with significant problems. Prolonged use of ART leads to severe side effects. The virus can develop resistance to antiretroviral drugs. Even with adequate control with ART a state of chronic inflammation persists which can lead to the development of serious complications. Moreover, although increased numbers of infected people have access to ART, coverage is far from ideal in the countries with the greatest need. In 2015, it was estimated that only 17 million people were receiving treatment, representing only 46% of the world's infected population. All the rest, belonging to low- and middle-income areas, were unable to access treatment.1 Obviously important here is the fact that ART is very expensive.
There is therefore a need for strategies to find a cure for HIV. Over the last ten years, a tremendous amount of effort has been made in this field and there have been some significant advances. The main barriers preventing the cure of HIV with antiretroviral therapy have been identified, progress has been made in the understanding of the therapeutic targets to which potentially eradicating drugs could be directed, integrative strategies have been proposed and clinical trials with various alternatives are underway. The aim of this review is to provide an update on the main advances in HIV, with particular emphasis on the obstacles to HIV eradication and the different strategies proposed, a number of which are presently being tested.
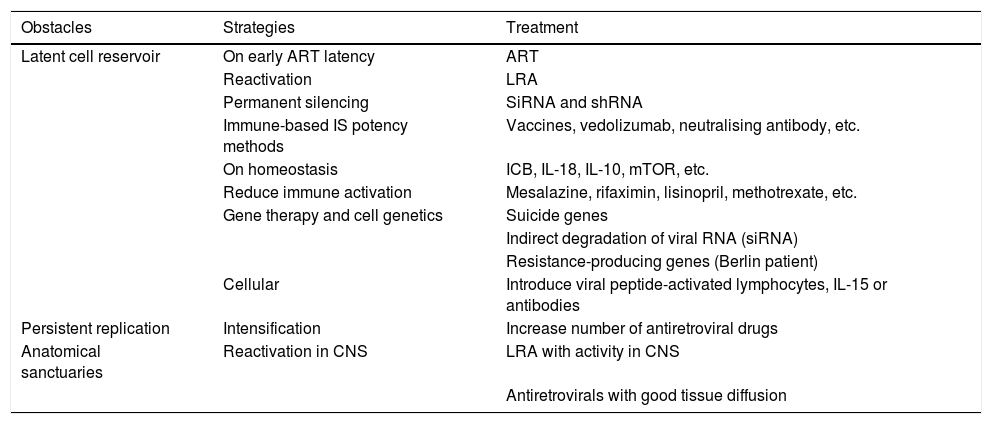
Obstacles to the eradication of HIVAt least three barriers have been identified which prevent HIV from being cured with the currently available weapons: the so-called latent cell reservoir; persistent HIV replication in spite of adequate antiretroviral therapy; and the accommodation of the virus in anatomical sanctuary sites. Although these three obstacles may not each have the same relative importance, there is no question that an eradication strategy should include measures to eliminate all of them. A further obstacle to be considered is the immune system's inability to recognise and eliminate HIV-infected cells (Table 1).
Mechanisms of HIV persistence.
| 1. Persistence of HIV in resting CD4+ T cells |
| 2. Activation of latently infected CD4+ T cells and infection of new cells |
| 3. Low-grade persistent replication |
| 4. HIV-induced clonal expansion |
| 5. Normal homeostasis without cytokine-mediated replication (IL-2, IL-7, IL-15) |
The cell reservoir is currently the main obstacle to the eradication of HIV-1 infection. It is composed mainly of latently infected resting memory CD4+ T cells that carry integrated and transcriptionally silent HIV-1 DNA. Within this population, it is thought that the cells which harbour latent HIV are mainly the central memory CD4 T cells (they express CD27 and CCR7) and the transitional memory CD4 T cells (they express CD27, but not CCR7). It also appears that, to a limited extent, naive T cells and, although less likely, multipotent stem cells in bone marrow may form part of the latent cell reservoir. Lastly, researchers have identified the so-called memory stem T cells (Tscm, T-lymphocyte memory stem cells), which represent the earliest and longest stage of memory T cells.2 Latently infected cells are widely distributed throughout the body, both in patients with no antiretroviral therapy and in treated patients with suppressed viral load. The cells can be detected in peripheral blood and, in particular, in lymphoid tissue.
Other cells may also form part of the reservoir.3 Macrophages and, probably, monocytes appear to be the main non-T cell reservoirs for HIV-1. Less clear is the role played by dendritic cells, regulatory T cells, NK cells, CD8 T cells and epithelial cells in the development of latency in the context of untreated HIV-1.4
As a common characteristic, latently infected cells have minimal expression of HIV-1 genes or their proteins, and are not recognised by the immune system because they have no surface proteins or markers to identify them as infected cells. One major problem is that there are no specific markers to identify latently infected cells, as a large part of the proviral DNA detected in these cells lacks the ability to replicate. Latently infected cells are known to have high levels of CD25 and CD32a was recently identified as a specific marker of CD4+ T cells latently infected with virus with replicative capacity.6
Establishment and maintenance of HIV latencyHIV-1 more easily infects activated CD4 T cells than memory and naive CD4 T cells, as naive cells do not have CCR5 co-receptors and their expression is reduced in memory CD4 T cells. CCR5 co-receptor expression is directly proportional to the degree of activation of the cell, so HIV-1 can directly infect cells with sufficient CCR5 density, including resting CD4 T cells, although it does not become integrated into the nucleus.
The reservoir becomes established during the primary infection and is very stable. After entering the cells, HIV-1 is transcribed into complementary DNA, translocated to the nucleus and integrated into the genome of the infected cell. While the CD4 T cell is activated, this proviral DNA will transcribe, initiating new replication cycles. Proviral DNA becomes integrated into transcriptionally active zones and around the genes that encode for host transcription factors7; the latter case gives it a selective advantage for clonal expansion. However, in some cells a latency state is established, and as the direct infection of memory T cells does not progress to integration, the only way the latent reservoir can be formed is by transition from the activation state of the infected CD4 T cell to the resting memory state, avoiding cell death.8 It is thought that one per million activated CD4 T cells are converted to resting cells and that, in peripheral blood, one in every million resting memory CD4 T cells harbours virus with competent replication, with there probably being a greater number in certain tissues.4
At least three mechanisms are involved in maintenance of the latently infected cell reservoir9:
- (1)
Suppression of transcription. It is now accepted that latency is largely maintained by the lack of sufficient levels in the nucleus of resting CD4 T cells of host transcription factors dependent on activation, such as NF-KB and NFAT, and of viral Tat proteins and host factors associated with Tat. Equally important is the role of positive transcription elongation factor (P-TEFb) and the micro-RNA in the silencing of the histone deacetylase and histone methyltransferase proviruses.9
- (2)
Homeostatic proliferation. Several interleukins, such as IL-7, are released at the time of cell activation, enhancing cell division. After the division two cells are generated, one of which enters into apoptosis and the other, containing the provirus in its genome, is kept alive and continues carrying out its usual functions. Recent studies show that cells with clonal expansion contain viruses with competent replication.10,11
- (3)
Negative regulators of T-cell activation. There are several transcription regulation pathways which start up when lymphocyte activation occurs. Recent studies have shown that cells with surface receptors PD-1, CTLA-4, TIGIT, TIM-3, LAG3, CD160 and CD2B4 are more likely to be in a latent state due to downregulation of replication.12
ART is highly effective in controlling viral replication. However, in the majority of patients treated correctly with ART, very low levels of viral RNA can be detected using ultrasensitive detection techniques. It has traditionally been maintained that low-level viraemia originates from reactivation of the small latent population and not the existence of constant viral replication. The absence of virus evolution in the isolates of patients who have been taking ART long-term has been used as the main argument against persistent viral replication.13
However, there are arguments that support the existence of new replication cycles in at least some groups of people receiving suppressive ART. In an intensification study with raltegravir, the persistence of virus replication was demonstrated when an increase in the levels of circular episomal DNA was found with 2-LTR, signifying inhibition of replication.14 We know from models that measure the dynamics of viral replication that the haplotypes of the viruses found in lymph nodes and in the blood are very similar, although there are some substantial genetic variations in response to powerful selective forces. Virus replication in infected cells in lymphoid tissues, where ART does not reach adequate concentrations, could establish the virus reservoir and allow trafficking to blood and other lymphoid tissues.15One study showed that it is not inevitable that the virus will generate resistance to ART because the low concentrations of antiretrovirals in anatomical sanctuaries are not enough to give it a defensive advantage against the drug.16
It has also been shown that follicular CD4 T cells (T helper cells in the lymph node) are inaccessible to activated CD8 T cells, which helps to perpetuate residual viral replication in the lymph nodes. In fact, the B-cell follicle is the site with the most active viral replication, because the lack of CD8 T cells allows the replication of HIV due to the lack of efficient T-cell response in this region.17
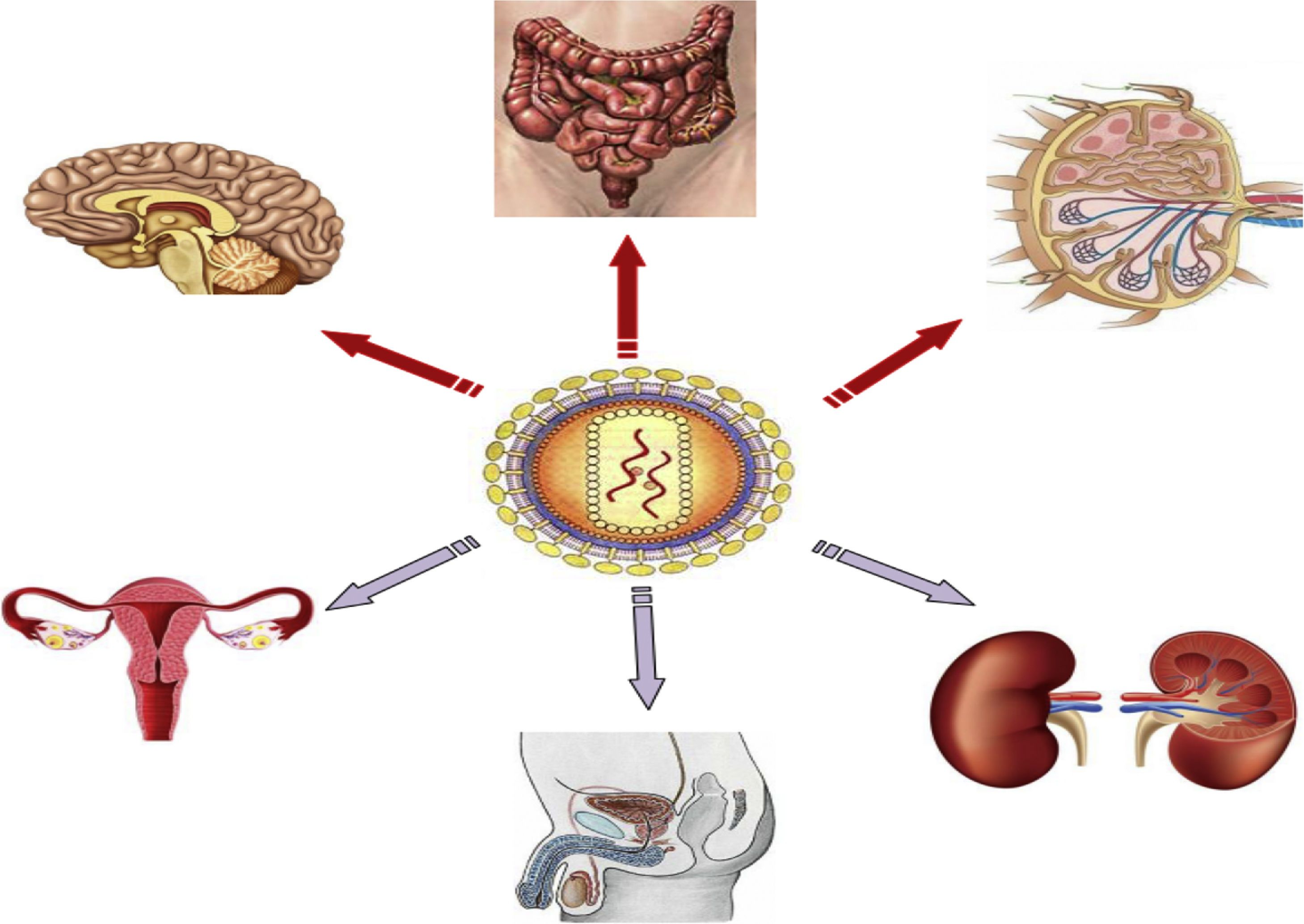
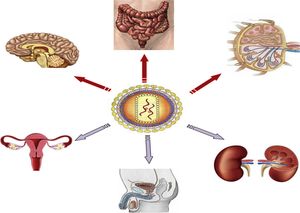
Anatomical sanctuariesThe main anatomical reservoirs of infected cells are in lymphoid tissue, such as that located in the genital tract and lungs, gut-associated lymphoid tissue (GALT) and non-lymphoid organs, such as the CNS and kidneys (Fig. 1). Of note is that CD4 T cells in peripheral blood only account for 2% of total body lymphocytes, while GALT houses 60% of total body lymphocytes, 40% of which are CD4 T cells.18 GALT is the most important anatomical sanctuary as its characteristics mean that it is not possible to obtain an adequate concentration of ART consistent throughout the tissue. CD4 T cells are also found in the lymph nodes and higher HIV RNA and DNA concentrations have been measured than those found in peripheral blood. The role of other anatomical sites (brain, male and female genital tract, kidneys and liver) as a reservoir of HIV is more unlikely.19
Strategies for eradicating HIVStrategies for eliminating the latent cell reservoirInterventions on latent HIVa. Reduction in the establishment of the reservoir or its size: early introduction of antiretroviral therapyThe proposal of starting ART early to help with the cure of HIV is based on the potential capacity to reduce the activation of T cells, increase the capacity of the immune system to control viral replication and reduce the size of viral reservoirs.20
Several studies in animals have shown that it is possible to reduce the latent cell reservoir by administering ART during the primary infection phase. However, ART is unable to prevent the establishment of latency.21,22 A number of studies in humans support the animal findings. The timing of establishment of the reservoir in humans is still not fully understood. It serves to illustrate that the administration of ART in the first hours after exposure to HIV (for example, a needle-stick injury in a healthcare worker) markedly reduces the risk of acquiring HIV-1, which suggests that ART is capable of eradicating the first infected cells after virus exposure.20 At the same time, it has been demonstrated that starting ART at ten days after the onset of symptoms of primary HIV-1 infection does not prevent the generation of latency, despite effective control of plasma viraemia; and that early ART, particularly prior to the detection of HIV immunoglobulin M, can reduce the number of long-lived central memory CD4 T cells, but those which are latently infected continue to persist.23
A number of studies point to the need for very early treatment to limit the size of the latent cell reservoir. In a Thai study, it was confirmed that among patients with acute infection treated early with ART, significant reductions in proviral DNA levels were only achieved during the earliest phase of acute infection24 compared to the later stages of primary infection.25
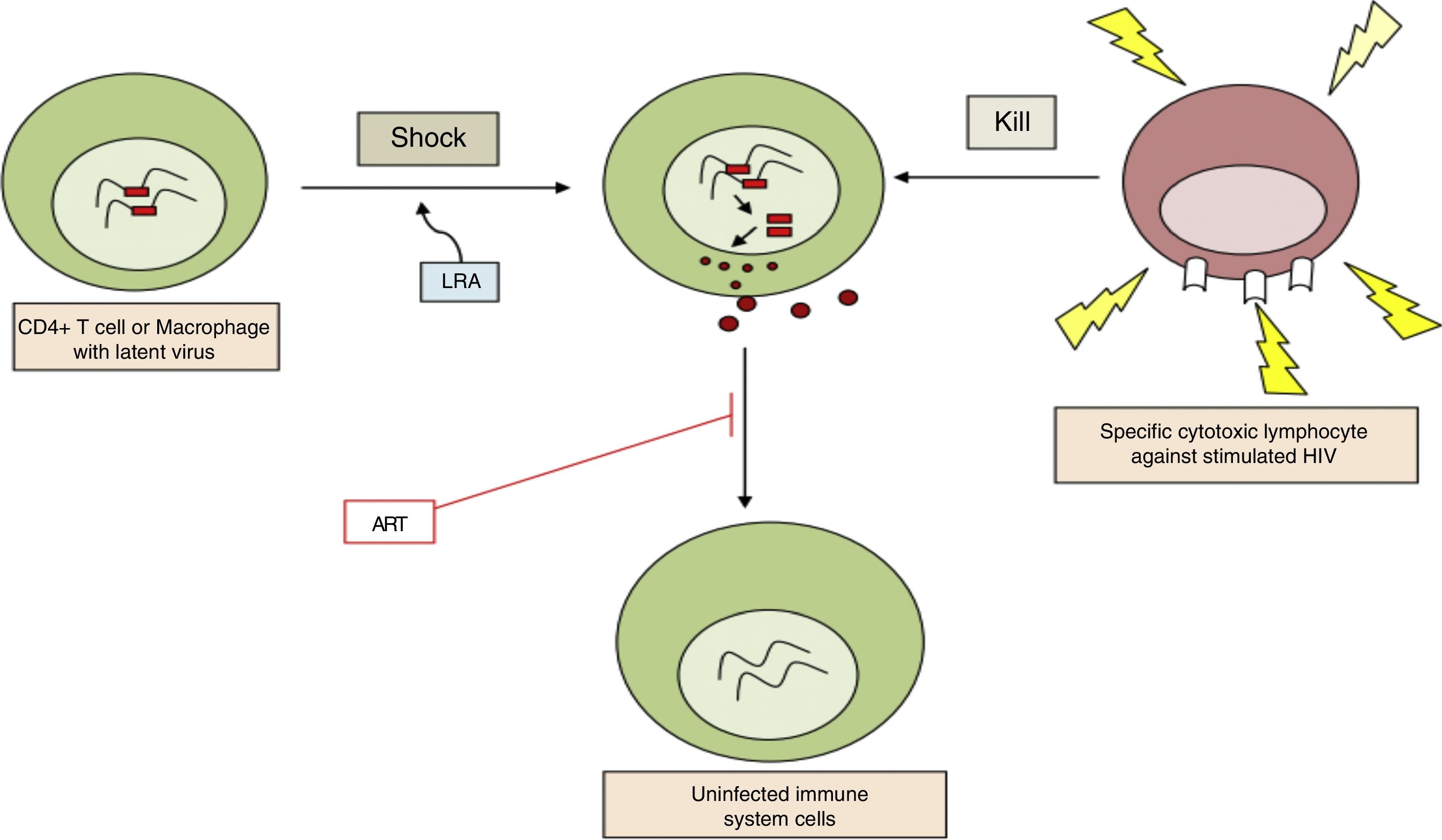
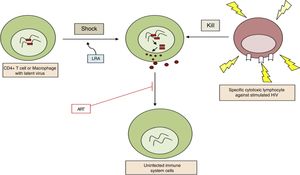
b. Reversal of established latency: pharmacological reactivationThe aim is to activate viral replication and produce cell division in latent infected cells. This is the first part of the “shock and kill” strategy where the latent infected cells are reactivated (“shock”), following which, as the viral antigens are now recognised by the immune system, the reactivated infected cells can be eliminated (“kill”) (Fig. 2). It was assumed that after reactivation the infected cell would undergo apoptosis. However, in vitro studies have shown that this is not the case with resting CD4 T cells.11
Latency-reversing agents are able to do this by various signalling pathways (Table 2).26 Overall, the current latency-reversing agents probably lack sufficient potency to reduce the reservoir. In clinical trials carried out in humans, the administration of isolated drugs had no effect on the rate of latent infected cells.27 Combinations of latency activators are therefore proposed, as reversal of the latency could be governed by stochastic mechanisms (some genes remain silenced even when there is maximum mitogenic stimulation). The synergistic effect between histone deacetylase inhibitors, methylation inhibitors, histone methyltransferase inhibitors, NF-kB activators and IL-7 has been demonstrated in vitro, but further studies are required.28
Latency-reversing agents. Mechanisms of action and reactivating intensity.
| Drugs | Mechanism of action | Intensity |
|---|---|---|
| NF-kB-inducing agents | ||
| Anti-CD3/CD28 | Activation of the T-cell receptor | ++++ |
| TNF-alpha | Activation of TNF receptor | ++ |
| Prostratin, PMA/ionomycin, bryostatin-1, Picolog | Activation of protein kinase C | +++ |
| HDAC inhibitors | Acetylation of histone and non-histone proteins | |
| Vorinostat | -HDAC class 1, 3, 4 and 6 and cell cycle controllers: they lower Myc and increase p21 -Reactive oxygen species: increase apoptosis -Angiogenesis: HIF alpha, low VEGF -Innate immunity, low pro-inflammatory cytokines, STAT5 hyperacetylation | ++ |
| Romidepsin (FK228) | HDAC 1, 2, 4 and 6 inhibitor | +++ |
| Panobinostat, givinostat and belinostat | Decrease in potency: LBH589, ITF2357, PXD101, SAHA, VPA | +++ |
| Sirtuin inhibitors | Acetylation of the non-histone proteins: Tat HIV, RelA p65, p53 and FOXO3a | Not tested |
| HMT inhibitors | ||
| BIX-01294 | G9a inhibitor (H3K9me2) | ++ |
| Chaetocin | SUV39H inhibitor (H3K9me3) | ++ |
| Cellular and pro-apoptotic differentiation molecules | ||
| Nutlin | -MDM2 blockade -Transcription of p53, activation of caspase | Not tested |
| Disulfiram, aphidicolin | Increase in oxidative stress and apoptosis | ++ |
| HMBA, dactinomycin, aclarubicin and cytarabine | Released P-TEFb associated with RNAP II that allows transcriptional elongation | + |
| Immune modulators | ||
| Anti-PD-1, CTLA-4, TIM-3 monoclonal antibody | Blockade of negative regulation receptors | Anti-PD1 (ACTG 5301) |
| Anti-PD-1 monoclonal antibody and interleukin 15 | STAT5, PI3k signalling, differentiation of the effector memory CD4 T cells | Not tested |
| Therapeutic CD4 T-cell vaccine and raltegravir | Reactivation of the virus in latently infected antigen-specific CD4 cells and limit the extension of the viral reservoir | Not tested |
| Synergistic combinations | ||
| Vorinostat and chaetocin, Vorinostat and prostratin | HMT inhibitor and HDAC inhibitor NF-kB inducer and HDAC inhibitor | +++ |
| HDAC inhibitor and anti-PD-1 | HDAC inhibitor and anti-PD-1 | Preclinical studies |
| Anti-PD-1 monoclonal antibody and interleukin 15, raltegravir and peptidomimetic | Differentiation of effector memory CD4 cells Integrase LEDGF/p75 inhibitor | Not tested |
ACTG: AIDS Clinical Trial Groups; CD4T: CD4 T cells; CTLA-4: cytotoxic T lymphocyte antigen-4; HDAC: histone deacetylase; HIF: hypoxia-inducible factor; HMBA: hexamethylene bisacetamide; HMT: histone methyltransferases; MDM2: murine double minute 2; PD-1: programmed cell death protein 1; RNAP II: RNA polymerase II; STAT: signal transducer and activator of transcription; TNF: tumour necrosis factor; VEGF: vascular endothelial growth factor.
In addition to the drugs that act on the above targets, others have been identified with different mechanisms. The administration of toll-like receptor 7 (TLR 7) agonists in an animal model was recently shown to activate latent HIV and deplete the reservoirs.29 At the post-transcriptional level, the modulation of some types of micro-RNA has also been effective in reactivating latent virus. Galectin-9 can also reactivate latent HIV-1 by binding to non-specific membrane receptors and, in addition, can enhance cytotoxic T lymphocyte (CTL) activity by binding to the TIM-3 receptor.30
c. Permanent silencing of latent HIVThis alternative strategy differs from that of “shock and kill” by trying to completely and irreversibly suppress the transcription of HIV. It would mean permanent silencing and a halt to virus production after discontinuation of ART. It has been possible to direct the siRNA or shRNA (short hairpin RNA) to the HIV-1 promoter region, inducing transcriptional silencing of the virus genes by inducing stable epigenetic modifications in the viral genome.31
Immunological methodsa. Increasing the capacity of the immune system to eliminate or control HIVInduction of transcription of the latent provirus by LRA would cause the expression of HIV-1 antigens, which would make it easier for the immune system to identify the infected cells and eliminate them.11,32,33 However, the viral cytopathic effect alone may not be enough to eliminate latent infected cells.
We know that constant antigenic stimulation during HIV-1 infection leads to chronic immune activation and subsequently to immune depletion. Cells with a specific response against HIV diminish or become dysfunctional, losing antiviral function and proliferative capacity.34 HIV ends up reducing the cytopathic function of the CD8 T cells and the depletion of the B cells causing an inadequate antibody response. Cell-mediated mechanisms for the elimination of the virus may therefore be insufficient.
The importance of cellular immunity in the control of reservoir size has been demonstrated. In an ex vivo study, Gag-specific HIV CD8 T cells isolated from elite controllers (people who control HIV replication naturally), but not from post-treatment controllers (those who control HIV replication after discontinuation of antiretroviral treatment, in general, started very early), were capable of killing latently infected CD4 T cells in which HIV was reactivated after the administration of vorinostat.11 It has also been discovered that the cytotoxic CD8 T cells with high multifunctionality targeted at the vulnerable regions in Gag are decisive in limiting the diversity and viral reservoir in HIV-infected patients who have protective HLA class I alleles.35
Therapeutic vaccinesThe aim of therapeutic vaccination would be to boost the immune system response in an already infected patient. Some vaccines have been shown to improve the specific immune response to HIV-1, but none has allowed the withdrawal of ART to be maintained. In non-human primate models, they have begun to use live CMV as a manipulated vector to stimulate a response to new and non-immunodominant epitopes, and have achieved good results.36 The use of amphiphilic peptides to direct antigens to the lymph nodes and induce a simultaneous immune response to two or more regions of HIV immunological vulnerability is still under investigation.37 Vaccines based on dendritic cells containing specific antigens (antigen-pulsed autologous dendritic cells)38 could increase the effective activation of CD8 T cells.
Neutralising antibodiesThe antibodies could reduce the degree of chronic inflammation or target latently infected cells that continue to express antigens spontaneously or after induction with LRA. The use of some specific neutralising monoclonal antibodies (mAbs) has achieved significant suppression of viraemia and even a considerable reduction in proviral DNA in peripheral blood, lymph nodes and gastric mucosa in animals, without subsequent viral rebound.39
In preclinical models, researchers have begun to use bispecific antibodies that stimulate virus production and simultaneously recognise and eliminate cells that express viruses. Although yet to be used in clinical practice, we have to recognise their potential to cause damage.40 Moreover, mAbs could also be used to reactivate the antibody-dependent cellular cytotoxicity which, through its non-MHC-dependent immune response, might recognise and eliminate reactivated infected cells. The mAb-dependent cellular cytotoxicity of HIV-specific antibodies binds to viral antigens on the surface of the infected cell and activates the innate immune response of natural killer cells or monocytes via the Fc receptor.41
Immune checkpoint blockade (ICB)This strategy also aims to increase host immune control. The most studied immune receptors in this field are PD-1, CTLA-4, LAG-3, TIM-3, TIGIT and 2B4, which are actually markers of functional exhaustion. They are mainly expressed in activated T cells.
The best known is PD-1, the ligands of which are PDL-1 and PDL-2, which are widely expressed in the tissues. PD-1 is the protein responsible for programmed cell death, and it plays an important role in immune depletion. When PD-1 binds with PDL-1, an inhibitory signal is produced in the antigen-dependent receptors, preventing the signal necessary for the proliferation, effective function and replication of HIV. When PD-1 or PDL-1 inhibitors release this inhibitory signal on T-cell activation receptors, this increases the sensitivity of the receptors to the antigens. Antibodies to PD-1 have been investigated. A phase I clinical trial conducted for this purpose had to be stopped prematurely due to the onset of severe adverse effects, mainly related to autoimmune phenomena, consistent with the severe toxicity observed in an animal model.42
Other strategiesVedolizumab is a human analogue of a monoclonal antibody targeted against the heterodimeric form of integrin α4β7 used in the treatment of inflammatory bowel diseases because it prevents the passage of lymphocytes into the gastrointestinal tissue.43 It has been discovered that both HIV-1 and SIV have a preference for infecting CD4 T cells with a higher level of integrin α4β7.44 A clinical trial in Rhesus macaques using a recombinant monoclonal antibody against integrin α4β7 showed the absence of viral rebound two years after administration of the antibody, with the apparent cure of the animals.18,45 The mechanism by which this effect was achieved remains unclear. A clinical trial with vedolizumab is currently underway in humans with chronic HIV-1 infection.
Immunotoxins are bifunctional chimeric proteins formed by a targeted portion, such as an antibody or a ligand, and a dominant efferent toxin. The administration of immunotoxin 3B3-PE38 in combination with ART showed reduced tissue levels of RNA in humanised mouse models46 greater than the reduction achieved with ART alone.
b. Act on homeostasis and T-cell dysfunctionThe aim is to reverse the chronic inflammation, with the hope that this may contribute to cure or remission. This could be achieved by blocking or stimulating different pathways, such as immunological checkpoints (PD-1, CLTA-4, TIGIT and others), indoleamine-pyrrole 2,3 dioxygenase (IDO), interleukin 18, mTOR, JAK/STAT, IL-10 and TGF-B. Activation and proliferation of T cells are controlled by a large number of signalling pathways, such as mTOR (mammalian target of rapamycin), signal transducer and activator of transcription 5 a (STAT5a) and O3a (forkhead box),47 and inhibition of these pathways might therefore make the latency permanent or reduce the size of the reservoir. This is the case of the immunosuppressant sirolimus (rapamycin), which, used as a single drug, has achieved low levels of cell-associated HIV DNA.
c. Reduction in immune activationThe aim of this strategy is to prevent the passage of activated CD4 T cells into the resting state, thus decreasing the likelihood of the latency increasing. This would be achieved by adding immunosuppressants to the ART.48 In clinical trials, drugs such as mesalamine, rifaximin, lisinopril and methotrexate have been used, but the results have not been very promising.
Gene and cell therapy(a) Gene therapyThe aim of the most studied application of gene therapy is to replace infected cells with a new population of HIV-1-resistant cells. The ideal cell population to intervene is stem cells. The concept test was demonstrated with a bone marrow transplant using stem cells from a compatible donor homozygous for the CCR5-delta32 deletion. Full remission was achieved and there has been no virus rebound since 2007 with no ART. This individual, known as the “Berlin patient”, became the first and only example of HIV eradication.49 Although the cure may have been produced by a combination of different factors (pre-transplantation regimen, graft versus host disease), the cells’ resistance to be infected by HIV because of the absence of the CCR5 co-receptor certainly played an important role. In fact, the next attempt at achieving cure in two patients (the so-called “Boston patients”) with allogeneic bone marrow transplantation from donors heterozygous for delta32 deletion failed.50 In another patient who had allogeneic transplantation of CCR5-Delta32 haematopoietic cells, all test data that might indicate viral infection are negative, but discontinuation of antiretroviral treatment to assess definitive cure is still pending (Düsseldorf patient).51
Motivated by the success of the Berlin patient, they began to make genetic modifications to the individual's own cells to provide those cells with resistance to HIV. The new techniques seek to inhibit or halt the expression of CCR5 by way of CCR5 gene silencing (knockdown) using three different types of enzymes: zinc finger nucleases (ZFN), transcription activator-like effector nucleases and cellular endonucleases (clustered regularly interspaced short palindromic repeats [CRISPR-Cas9/]).52
Gene therapy has also been used to enhance the ability of T cells to eliminate HIV-infected cells. In cancer studies, genetic modification has been used to express chimeric antigen receptors with improved antigen specificity. This has also been adapted in strategies against HIV, genetically modifying the peripheral blood cells to have a molecularly cloned T-cell receptor (TCR) which directs the cell to recognise the viral antigen.53 The toxic potential of these strategies is still unknown.
(b) Cell therapiesConsist of transferring HIV-1-specific CTL. CTL stimulated with Gag have been found to be much more effective in cellular models of latency than unstimulated CD8 T cells.11 Many studies have shown that exposing T cells to multiple peptides from different HIV-1 antigens enables them to increase the elimination of latent infected cells that have been reactivated ex vivo.54
There are also cytotoxic immune cells other than CTL which could be used to eliminate infected cells and that act by different mechanisms, such as natural killer cells, killer cells activated by lymphokines and T cells. IL-1555 or monoclonal antibodies are usually used to improve the effector response of the cells, and in the case of natural killer cells, the antibody-dependent cellular cytotoxicity pathway is used.56
Strategies to eliminate persistent viral replication: intensification of antiretroviral treatmentIntensification consists of increasing the dose of established ART or adding more potent agents to the ART regimen with the aim of reducing the size of the reservoir by completely suppressing viral replication. An attempt is made to achieve therapeutic concentrations in anatomical sanctuaries, where residual viral replication persists. Although overall, intensification treatment has not been shown to reduce the size of the reservoir or prevent viral rebound after withdrawal of ART,57 results have been published suggesting that this strategy may contribute to the elimination of the reservoir. The HIV-1 integrase inhibitor, raltegravir,14 and the CCR5 co-receptor antagonist, maraviroc,58 have been used for this purpose.
Strategies to eliminate anatomical reservoirsThe central nervous system (CNS) is the only area to have been the subject of research in this regard. It is thought that the brain acts as a reservoir for HIV during ART, isolated and independent from all the other reservoirs.59 There are suggestions of potential dangers from reactivation of the latent virus in the CNS. The increased activation of the immune system in the brain could lead to reactivation of the latent reservoirs followed by an exacerbated and damaging inflammatory response, even in the presence of ART. Taking into account that the CNS has macrophages with persistent competent replication, monitoring viral activation in CSF and residual viraemia could be key when implementing eradication strategies.60
Functional cure or HIV eradicationThere are currently two quite distinct approaches to curing HIV, what is known as functional cure and eradication. In a patient who is not taking ART, functional cure is defined as the absence of detectable viral replication indefinitely or for a set period of time. This means control of host-mediated viral replication, such that, without ART, immune function would be restored and stabilised, HIV-1-induced inflammation reduced and plasma viral load maintained at very low levels, reducing the risk of transmitting the virus. In this scenario, even though competent replication of HIV persisted, the patient would be able to discontinue ART without risk. Functional cure has been well documented naturally in elite controllers and, through ART, in post-treatment controllers, as well as in the examples of partial cure: “Mississippi girl” and “Boston patients”.
For its part, eradication or “sterilising” cure is defined as the complete elimination of all viruses with competent replication in infected patients. Given this context, there is no chance of viral rebound when ART is discontinued.27 Although this type of cure was achieved in the “Berlin patient”, it is considered a less viable objective.
All the proposed strategies for achieving functional cure of HIV infection analysed in the previous sections are set out in Table 3.
Proposed strategies for overcoming the obstacles identified in the functional cure for HIV.
| Obstacles | Strategies | Treatment |
|---|---|---|
| Latent cell reservoir | On early ART latency | ART |
| Reactivation | LRA | |
| Permanent silencing | SiRNA and shRNA | |
| Immune-based IS potency methods | Vaccines, vedolizumab, neutralising antibody, etc. | |
| On homeostasis | ICB, IL-18, IL-10, mTOR, etc. | |
| Reduce immune activation | Mesalazine, rifaximin, lisinopril, methotrexate, etc. | |
| Gene therapy and cell genetics | Suicide genes | |
| Indirect degradation of viral RNA (siRNA) | ||
| Resistance-producing genes (Berlin patient) | ||
| Cellular | Introduce viral peptide-activated lymphocytes, IL-15 or antibodies | |
| Persistent replication | Intensification | Increase number of antiretroviral drugs |
| Anatomical sanctuaries | Reactivation in CNS | LRA with activity in CNS |
| Antiretrovirals with good tissue diffusion |
ART: antiretroviral therapy; CNS: central nervous system; ICB: immune checkpoint blockers; LRA: latency-reversing agent; shRNA: short hairpin RNA; siRNA: short interfering RNA.
We have identified the obstacles to cure and documented the inadequacy of the strategies adopted so far. However, we have also shown that cure is possible and that, whether naturally or using different treatments, HIV remission can be achieved without ART.
Having laboratory methods that enable us to quantify the size of the cell reservoir and identify the latently infected cells are two of the main issues the scientific community urgently needs to resolve, in order to allow us to determine the eradication capacity of any strategy we wish to test. Without this, withdrawal of antiretroviral therapy remains the only way to check the success of the different types of treatment.
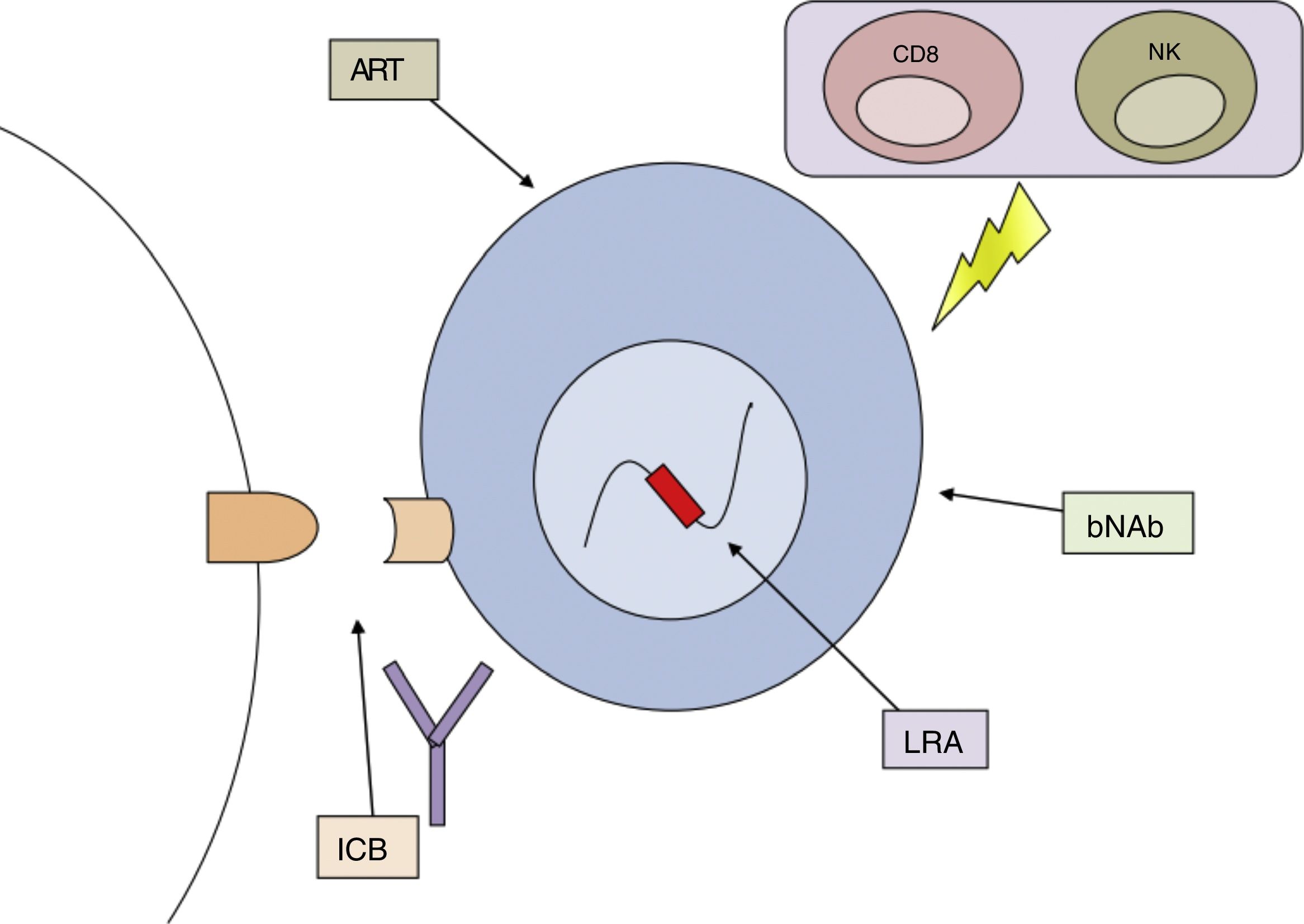
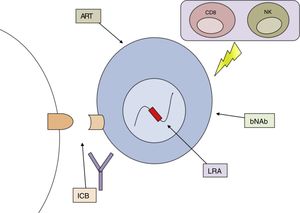
No method has yet been identified which has consistently achieved reservoir reduction or achieved partial or complete remission. The failure of different measures applied in isolation has led to the combined or sequential use of several strategies directed against different mechanisms that allow HIV to persist in the organism being proposed as the best option for achieving the cure of this disease (Fig. 3).
FundingThis study was funded by the project Red de Investigación en SIDA (RIS) [AIDS Research Network]RD16/0025/0001, which is part of the Spanish National Scientific Research, Development, and Technological Innovation Plan, and co-funded by the Subdirectorate-General for Assessment of the Instituto de Salud Carlos III (Carlos III Health Institute) and the European Regional Development Fund (ERDF).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Muñoz J, Moreno S. Estrategias de curación de la infección por VIH. Enferm Infecc Microbiol Clin. 2019;37:265–273.