Our objective was to determine whether there is a cut-off in the needleless connectors’ (NCs) cultures that when combined with skin cultures it was as efficient as conventional superficial cultures to rule-out catheter colonization (CC) and catheter-related bloodstream infection (CRBSI).
MethodsDuring 10 months, we collected samples and then we analyzed the validity values of skin+NCs cultures for CC and CRBSI considering the best cut-off showing at least >90% of specificity to have a high negative predictive value using a ROC curve.
ResultsWe collected a total of 167 catheters. The optimal cut-off of NCs culture was 1000cfu/NC. The validity values for CC and CRBSI combining skin cultures and NCs cultures using the selected cut-off were, respectively: S, 42.9%/16.7%; SP, 83.6%/75.8%; PPV, 27.3%/2.5%; and NPV, 91.0%/96.0%.
ConclusionsThe combination of skin cultures and quantitative NCs cultures could be used for ruling-out CC and CRBSI.
Nuestro objetivo fue determinar si existe un punto de corte en los cultivos de conectores sin aguja (NC) que, cuando se combina con cultivos de piel, sea tan eficiente como los cultivos superficiales convencionales para descartar colonización de catéter (CC) y bacteriemia relacionada con el catéter (BRC).
MétodosDurante 10 meses se coleccionaron muestras, y después se analizaron los valores de validez de los cultivos de piel + NC para CC y BRC considerando el mejor punto de corte aquel que mostrara al menos >90% de especificidad para tener un alto valor predictivo negativo usando una curva ROC.
ResultadosSe estudiaron un total de 167 catéteres. El punto de corte óptimo del cultivo de NC fue de 1.000ufc/NC. Los valores de validez para CC y BRC combinando cultivos de piel y cultivos de NC utilizando el punto de corte seleccionado fueron, respectivamente: S: 42,9/16,7%; ES: 83,6/75,8%; VPP: 27,3/2,5% y VPN: 91,0/96,0%.
ConclusionesLa combinación de cultivos de piel y cultivos cuantitativos de NC podría usarse para descartar CC y BRC.
The use of conservative diagnostic methods to detect catheter colonization (CC) and catheter-related bloodstream infection (C-RBSI) are of high importance in the clinical management of Major Heart Surgery (MHS) patients.1 Conventional superficial cultures (from the skin and hubs) demonstrated to have a high negative predictive value (90.4–96.7), for both colonization and C-RBSI, allowing to maintain catheters and to find another source of infection.2–5 However, hub cultures require rubbing inside the catheter lumen, which may be a risk for bacteraemia because the biofilm can be dislodged.6,7 Therefore, we demonstrated in previous studies that combining skin cultures with cultures of withdrawn needleless connectors (NCs) was an alternative and safer method to rule out CC and C-RBSI, showing no inferiority to conventional superficial culture.8–10
The aim of the present study was to assess whether there is a cut-off in the NCs culture that when combined with skin cultures was as efficient as conventional superficial cultures to rule-out CC and C-RBSI in patients admitted to MHS-intensive care unit (MHS-ICU).
MethodsHospital setting and patientsOur institution is a general referral hospital with 1550 beds and approximately 50,000 admissions/year. More than 500 MHS procedures are performed annually in the Department of Cardiovascular Surgery, which is a large referral unit.
Study designWe performed an ecological prospective study and included the patients on the MHS-ICU when a CVC remained in place ≥7 days after insertion.
At catheter withdrawal, simultaneous superficial samples (from the skin surrounding the catheter insertion site and from the inside of the hubs) and NCs (CLAVE™ systems, ICU Medical, Inc., San Clemente, CA, USA) were obtained.
The skin samples were obtained by lifting the dressing and rubbing the area around the insertion site (in a 3-cm radius) with dry cotton swab. The inner hub samples were obtained using alginate swabs that were introduced into the hub and rubbed repeatedly against its inner surface (1 swab per hub).
Superficial cultures were processed following standard semi-quantitative microbiological techniques.
Catheter tips were cultured using the roll-plate (Maki) technique and sonication onto a blood agar plate.
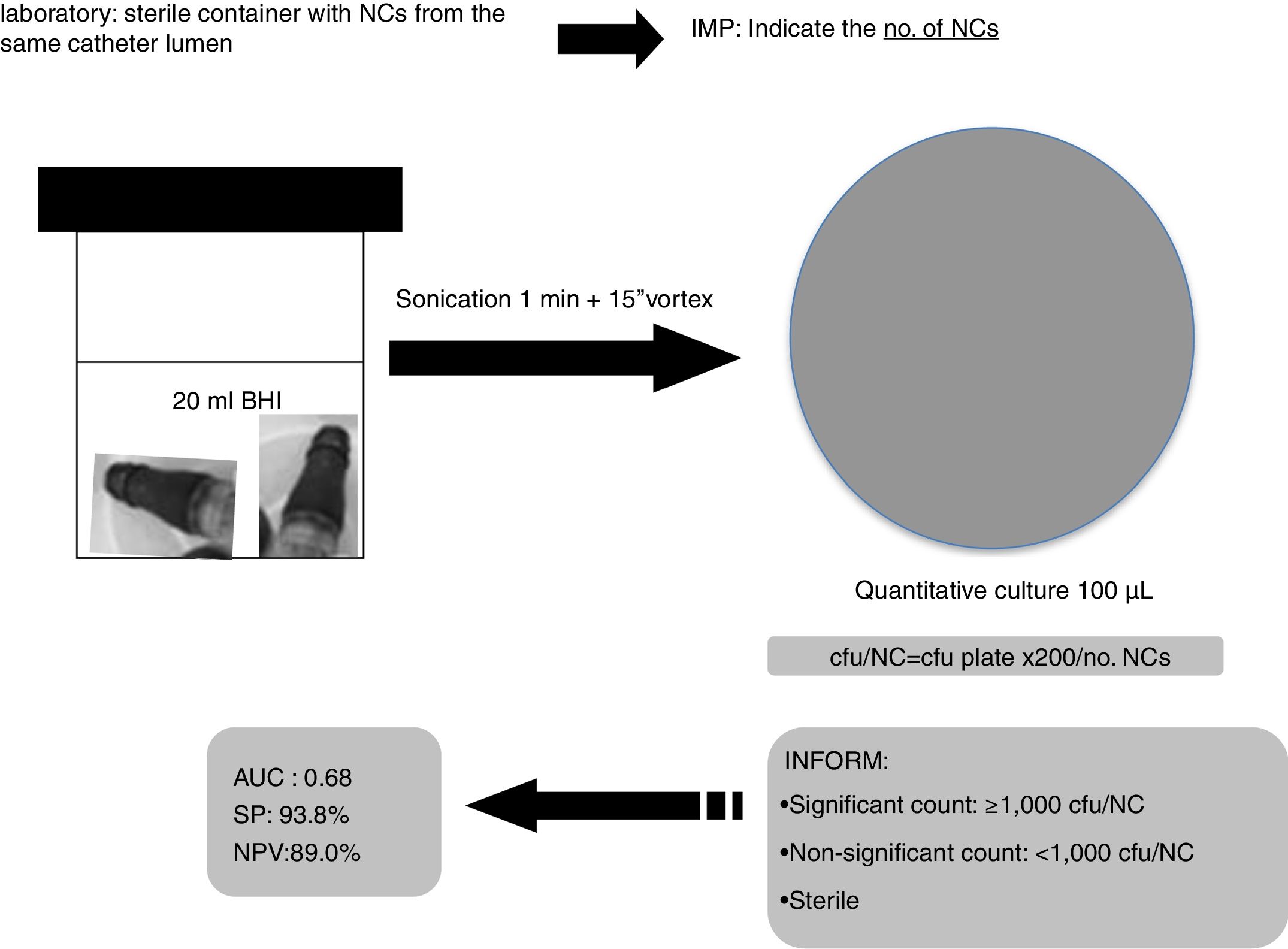
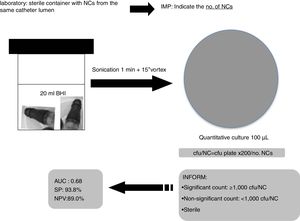
All groups of NCs belonging to a single catheter lumen (hub) were sonicated (1min) together into 20ml of BHI and 100μl were cultured onto blood agar plates and incubated for 24–48h at 37°C. The number of colony forming units (cfu) was counted for each set of cultures and the no. of cfu/NC was calculated (cfu/plate×200/no. NCs). We considered the lumen colonized when ≥1 culture was positive. The number of cultured NCs varied depending on the number of lumens in each catheter (1–5 lumens).
The gold standard to confirm catheter colonization was positivity of the catheter tip culture either by the semiquantitative Maki technique or by the sonication method.
We analyzed the validity values of skin and NCs’ cultures for CC and C-RBSI considering the best cut-off showing >90% of specificity to have a high negative predictive value using a ROC curve.
The microorganisms recovered were fully identified using standard microbiological methods.
We also used a pre-established protocol to record patient characteristics, underlying diseases, comorbidity conditions, severity-of-illness scores, such as acute physiology and chronic health evaluation II (APACHE II) scores, the maximum severity reached before catheter withdrawal, and microbiological data on blood cultures.
EthicsThe Ethics Committee of our institution (Hospital Gregorio Marañon) approved the study (MICRO, HGUGM. 2015-083) and written informed consent was obtained from the study participants.
DefinitionsCatheter tip colonizationIsolation of either ≥15cfu/plate with the semiquantitative Maki technique or ≥100cfu/segment with the sonication method.
Skin and hub colonizationIsolation of ≥15cfu/plate in semiquantitative culture.
We considered an episode of C-RBSI to be confirmed when the same microorganism was isolated both from peripheral blood cultures and from the catheter tip.
To calculate the validity values for NCs for predicting catheter colonization, we considered positive NCs those with the same microorganism(s) as isolated from the catheter tip.
Statistical analysisValues are expressed as the mean (SD) or median (IQR) for continuous variables and as percentages, with the 95% confidence interval (95% CI), when applicable, for categorical variables. Categorical variables were evaluated using the chi-square test or the two-tailed Fisher exact test. Statistical significance was set at p<0.05 (two-tailed). We calculated the validity values of the superficial culture (skin+hubs) and skin+NCs culture by comparing them with the gold standard of CC. The sensitivity, specificity, and positive and negative predictive values, with 95% CI, were calculated using EPIDAT 3.1. Finally, to assess a point of count of the NCs we conducted a ROC curve to determine the optimal cut-off to classify colonized NCs.
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp, Armonk, NY, USA).
ResultsDuring 10 months, 309 patients with 609 catheters were admitted in ICU-MHS. We finally included in the study 105 (34.0%) patients who had 167 (27.4%) central catheters inserted for ≥7days.
A total of 2166 cultures were made (167 tips, 251 skins, 874 hubs and 874 groups of NCs).
The mean (SD) age was 68 (60.5–77.0) years. The main underlying conditions of the patients were congestive heart failure (86.7%) and diabetes mellitus (26.7%). The median (QIR) APACHE II at inclusion, and EuroScore were 8.0 (6.5–10.0) and 8.0 (5.5–10.0), respectively.
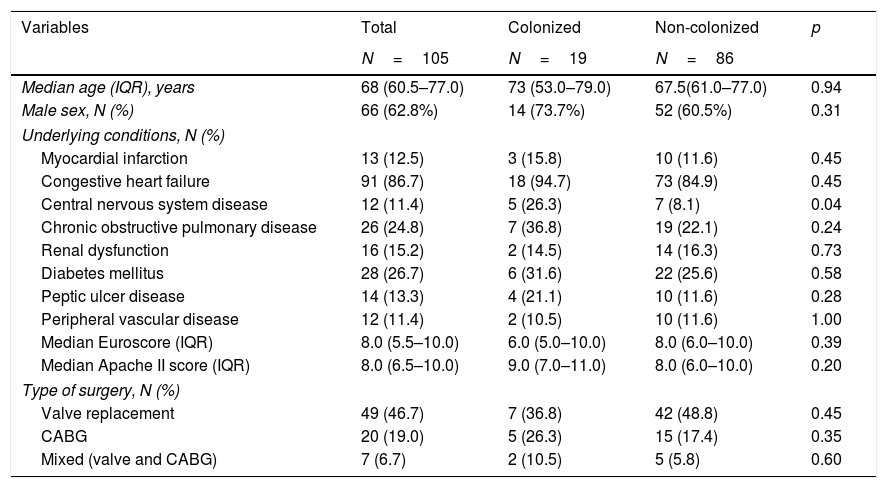
No significant differences in the underlying conditions and situation of the populations between colonized and non-colonized catheters were obtained (Table 1).
Main patients’ characteristics.
| Variables | Total | Colonized | Non-colonized | p |
|---|---|---|---|---|
| N=105 | N=19 | N=86 | ||
| Median age (IQR), years | 68 (60.5–77.0) | 73 (53.0–79.0) | 67.5(61.0–77.0) | 0.94 |
| Male sex, N (%) | 66 (62.8%) | 14 (73.7%) | 52 (60.5%) | 0.31 |
| Underlying conditions, N (%) | ||||
| Myocardial infarction | 13 (12.5) | 3 (15.8) | 10 (11.6) | 0.45 |
| Congestive heart failure | 91 (86.7) | 18 (94.7) | 73 (84.9) | 0.45 |
| Central nervous system disease | 12 (11.4) | 5 (26.3) | 7 (8.1) | 0.04 |
| Chronic obstructive pulmonary disease | 26 (24.8) | 7 (36.8) | 19 (22.1) | 0.24 |
| Renal dysfunction | 16 (15.2) | 2 (14.5) | 14 (16.3) | 0.73 |
| Diabetes mellitus | 28 (26.7) | 6 (31.6) | 22 (25.6) | 0.58 |
| Peptic ulcer disease | 14 (13.3) | 4 (21.1) | 10 (11.6) | 0.28 |
| Peripheral vascular disease | 12 (11.4) | 2 (10.5) | 10 (11.6) | 1.00 |
| Median Euroscore (IQR) | 8.0 (5.5–10.0) | 6.0 (5.0–10.0) | 8.0 (6.0–10.0) | 0.39 |
| Median Apache II score (IQR) | 8.0 (6.5–10.0) | 9.0 (7.0–11.0) | 8.0 (6.0–10.0) | 0.20 |
| Type of surgery, N (%) | ||||
| Valve replacement | 49 (46.7) | 7 (36.8) | 42 (48.8) | 0.45 |
| CABG | 20 (19.0) | 5 (26.3) | 15 (17.4) | 0.35 |
| Mixed (valve and CABG) | 7 (6.7) | 2 (10.5) | 5 (5.8) | 0.60 |
IQR, interquartile range; CABG, coronary artery bypass grafting.
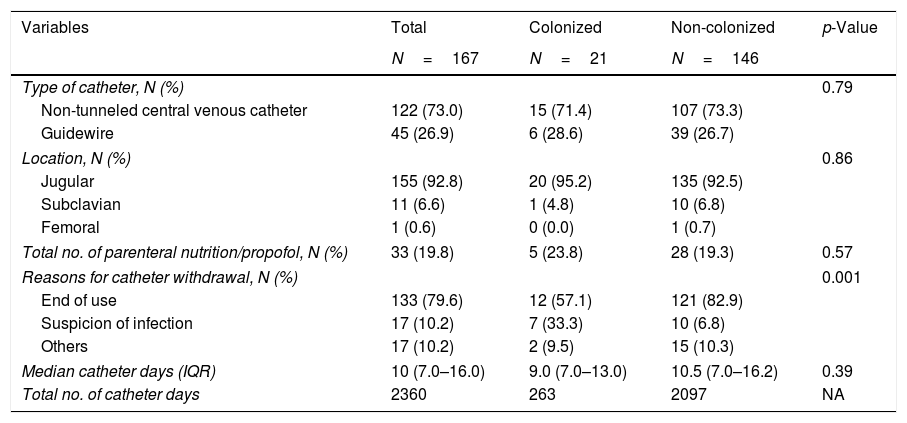
The main reason for catheter withdrawal was end of use (79.6%), followed by suspicion of infection (10.2%), and other reasons (10.2%). Other patient and catheter data are detailed in Table 2.
Main catheters’ characteristics.
| Variables | Total | Colonized | Non-colonized | p-Value |
|---|---|---|---|---|
| N=167 | N=21 | N=146 | ||
| Type of catheter, N (%) | 0.79 | |||
| Non-tunneled central venous catheter | 122 (73.0) | 15 (71.4) | 107 (73.3) | |
| Guidewire | 45 (26.9) | 6 (28.6) | 39 (26.7) | |
| Location, N (%) | 0.86 | |||
| Jugular | 155 (92.8) | 20 (95.2) | 135 (92.5) | |
| Subclavian | 11 (6.6) | 1 (4.8) | 10 (6.8) | |
| Femoral | 1 (0.6) | 0 (0.0) | 1 (0.7) | |
| Total no. of parenteral nutrition/propofol, N (%) | 33 (19.8) | 5 (23.8) | 28 (19.3) | 0.57 |
| Reasons for catheter withdrawal, N (%) | 0.001 | |||
| End of use | 133 (79.6) | 12 (57.1) | 121 (82.9) | |
| Suspicion of infection | 17 (10.2) | 7 (33.3) | 10 (6.8) | |
| Others | 17 (10.2) | 2 (9.5) | 15 (10.3) | |
| Median catheter days (IQR) | 10 (7.0–16.0) | 9.0 (7.0–13.0) | 10.5 (7.0–16.2) | 0.39 |
| Total no. of catheter days | 2360 | 263 | 2097 | NA |
IQR, interquartile range.
The overall catheter tip colonization rate was 12.6% (21/167) and 6 episodes of C-RBSI (2.5 episodes/1000 catheter days) were confirmed. Table 3 shows the microorganisms isolated from the colonized central venous catheters.
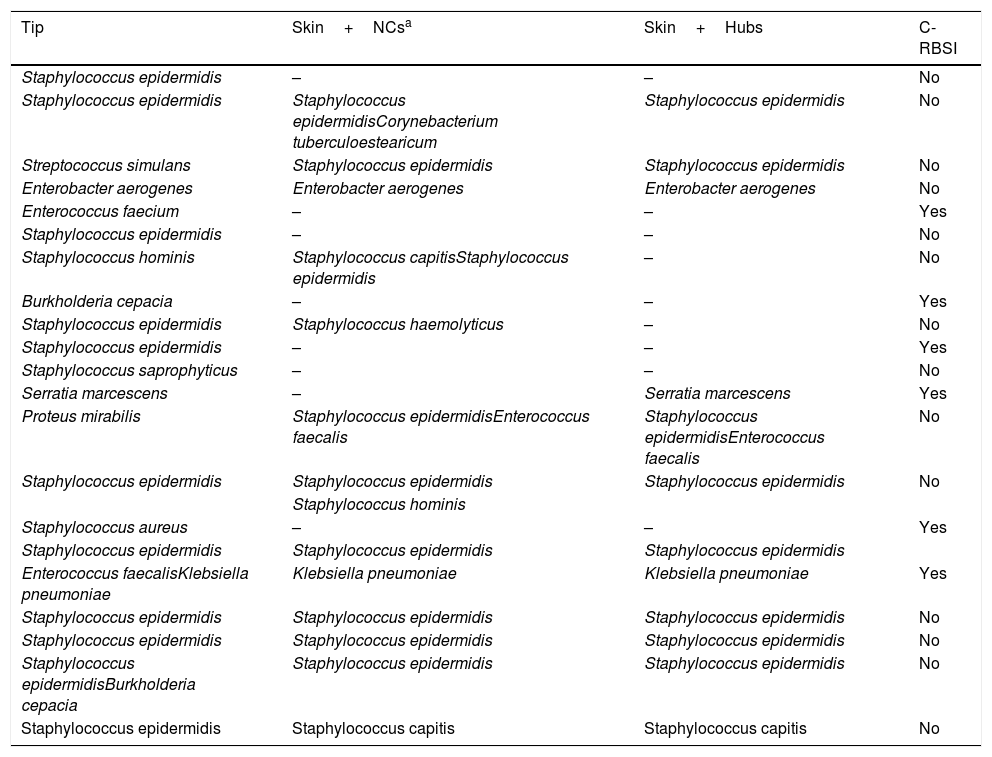
Microorganisms isolated in colonized catheters.
| Tip | Skin+NCsa | Skin+Hubs | C-RBSI |
|---|---|---|---|
| Staphylococcus epidermidis | – | – | No |
| Staphylococcus epidermidis | Staphylococcus epidermidisCorynebacterium tuberculoestearicum | Staphylococcus epidermidis | No |
| Streptococcus simulans | Staphylococcus epidermidis | Staphylococcus epidermidis | No |
| Enterobacter aerogenes | Enterobacter aerogenes | Enterobacter aerogenes | No |
| Enterococcus faecium | – | – | Yes |
| Staphylococcus epidermidis | – | – | No |
| Staphylococcus hominis | Staphylococcus capitisStaphylococcus epidermidis | – | No |
| Burkholderia cepacia | – | – | Yes |
| Staphylococcus epidermidis | Staphylococcus haemolyticus | – | No |
| Staphylococcus epidermidis | – | – | Yes |
| Staphylococcus saprophyticus | – | – | No |
| Serratia marcescens | – | Serratia marcescens | Yes |
| Proteus mirabilis | Staphylococcus epidermidisEnterococcus faecalis | Staphylococcus epidermidisEnterococcus faecalis | No |
| Staphylococcus epidermidis | Staphylococcus epidermidis | Staphylococcus epidermidis | No |
| Staphylococcus hominis | |||
| Staphylococcus aureus | – | – | Yes |
| Staphylococcus epidermidis | Staphylococcus epidermidis | Staphylococcus epidermidis | |
| Enterococcus faecalisKlebsiella pneumoniae | Klebsiella pneumoniae | Klebsiella pneumoniae | Yes |
| Staphylococcus epidermidis | Staphylococcus epidermidis | Staphylococcus epidermidis | No |
| Staphylococcus epidermidis | Staphylococcus epidermidis | Staphylococcus epidermidis | No |
| Staphylococcus epidermidisBurkholderia cepacia | Staphylococcus epidermidis | Staphylococcus epidermidis | No |
| Staphylococcus epidermidis | Staphylococcus capitis | Staphylococcus capitis | No |
NC, needleless connector; C-RBSI, catheter-related bloodstream infection.
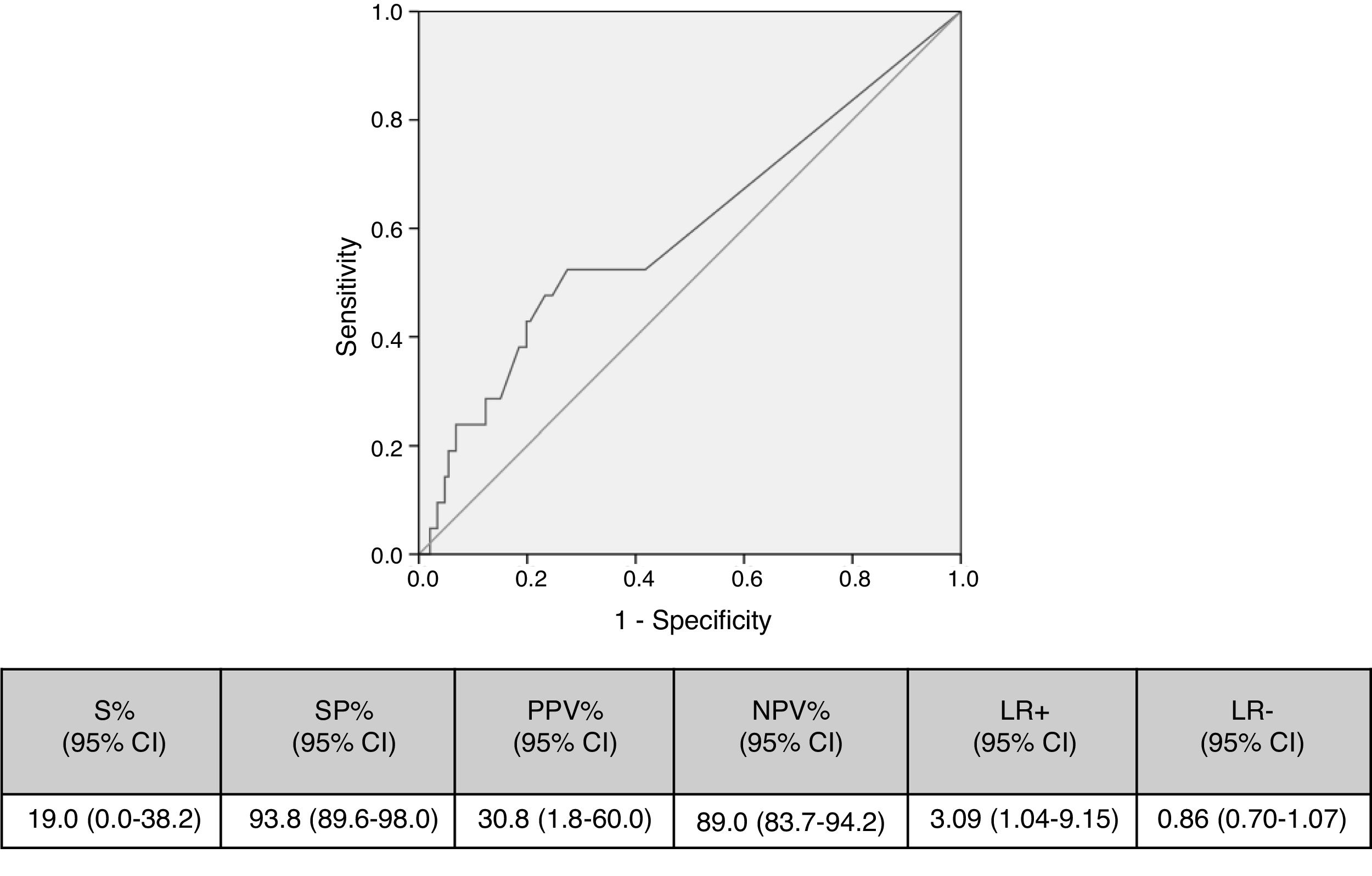
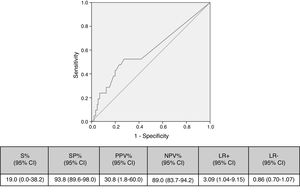
Cut-off points of 100cfu, 500cfu and 1000cfu were determined. As show in Fig. 1, a threshold value counts shows a sensitivity of 19% and specificity of 94%; if the cut-off of >1000cfu was selected; ROC curve 0.6 (0.45–0.74; IC). Although the auc is not very good, it is the best to have a high VPN.
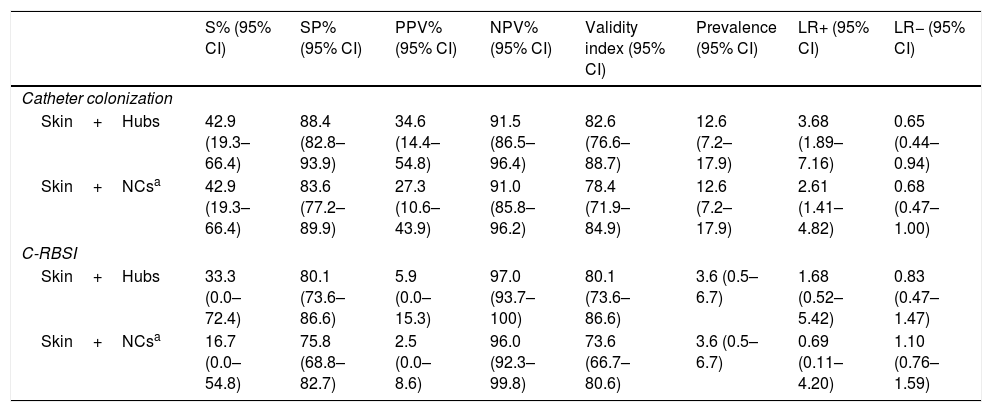
Table 4 shows the validity values for skin+NCs’ cultures using the selected cut-off and conventional superficial cultures (skin+hubs) for prediction of CC and C-RBSI. Skin+NCs’ cultures had 42.9% sensitivity and a 91.0% negative predictive value for CC compared with 42.9% and 91.5% for conventional superficial cultures. The validity values for skin+NCs’ cultures using the selected cut-off and conventional superficial cultures for C-RBSI were, respectively: sensitivity, 16.7%/33.3%; specificity, 75.8%/80.1%; positive predictive value, 2.5%/5.9%; and negative predictive, 96.0%/97.0% (Table 4).
Validity values of conventional superficial cultures and skin+NCs’ cultures for prediction of catheter colonization and C-RBSI.
| S% (95% CI) | SP% (95% CI) | PPV% (95% CI) | NPV% (95% CI) | Validity index (95% CI) | Prevalence (95% CI) | LR+ (95% CI) | LR− (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Catheter colonization | ||||||||
| Skin+Hubs | 42.9 (19.3–66.4) | 88.4 (82.8–93.9) | 34.6 (14.4–54.8) | 91.5 (86.5–96.4) | 82.6 (76.6–88.7) | 12.6 (7.2–17.9) | 3.68 (1.89–7.16) | 0.65 (0.44–0.94) |
| Skin+NCsa | 42.9 (19.3–66.4) | 83.6 (77.2–89.9) | 27.3 (10.6–43.9) | 91.0 (85.8–96.2) | 78.4 (71.9–84.9) | 12.6 (7.2–17.9) | 2.61 (1.41–4.82) | 0.68 (0.47–1.00) |
| C-RBSI | ||||||||
| Skin+Hubs | 33.3 (0.0–72.4) | 80.1 (73.6–86.6) | 5.9 (0.0–15.3) | 97.0 (93.7–100) | 80.1 (73.6–86.6) | 3.6 (0.5–6.7) | 1.68 (0.52–5.42) | 0.83 (0.47–1.47) |
| Skin+NCsa | 16.7 (0.0–54.8) | 75.8 (68.8–82.7) | 2.5 (0.0–8.6) | 96.0 (92.3–99.8) | 73.6 (66.7–80.6) | 3.6 (0.5–6.7) | 0.69 (0.11–4.20) | 1.10 (0.76–1.59) |
S, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; CI, confidence interval; NA, not applicable; NCs, needleless connectors; C-RBSI, catheter-related bloodstream infection.
Our study demonstrates that the combination of skin and NCs’ cultures considering a new cut-off had the same validity values as conventional skin and hub cultures to rule out CC and C-RBSI.
Patients admitted to MHS-ICU are at high risk for CC and, consequently for C-RBSI. When there is suspicion of C-RBSI it is important to achieve a rapid diagnosis for a proper management of the patient.11,12 The actual guidelines for the diagnosis of catheter-related infections recommend performing conservative diagnostic methods when there is suspicion of C-RBSI, such as superficial cultures from skin and hubs.13 These cultures have demonstrated their efficacy to rule out C-RBSI in several populations.1–5 However, hub cultures require rubbing a swab inside the catheter lumen that can dislodge the biofilm into the bloodstream.6,7 In order to reduce this risk, our study group have recently reported an alternative and safer diagnostic procedure with similar validity values to rule out CC and C-RBSI based on culturing the sonicate of withdrawn NCs. This was assessed in central venous systems from MHS-ICU patients.8–10 However, in these studies NCs’ were processed considering a positive culture of the sonicate when any number of cfu was counted (qualitative culture), which implied a high number of false positive results. In the present study we solved this issue by considering a cut-off (1000cfu/NC) in the NCs’ culture of the sonicate which demonstrated that, when combined with skin cultures, the validity values for CC and C-RBSI were similar to that obtained with conventional superficial cultures from skin and hubs.
Based on our data, we suggest to Microbiology laboratories a new proposal for the processing and interpretation of NCs’ cultures by sonication for a proper management in the diagnosis of catheter colonization and C-RBSI (Fig. 2).
ConclusionDespite the combination of skin cultures and quantitative NCs’ cultures did not show good sensitivity, they could be used as a conservative diagnostic procedure for ruling-out catheter colonization and C-RBSI when no catheter withdrawal is possible.
FundingM. Guembe is supported by the Miguel Servet Program (ISCIII-MICINN, MS13/00268) from the Health Research Fund (FIS) of the Carlos III Health Institute (ISCIII), Madrid, Spain. Beatriz Alonso is supported by the Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid and Fondo Social Europeo (PEJ15/BIO/AI-0406). The study was partially financed by the European Regional Development Fund (FEDER) “A way of making Europe” (PI18/00045), by Ciber de Enfermedades Respiratorias (CIBERES), and by grants from the Instituto de Investigación Sanitaria Gregorio Marañón (II-PI-ENF-2016-2).
Conflict of interestsNone of the authors have conflicts of interest to declare.
We thank Thomas O’Boyle for his help on the preparation of the manuscript.