Anal cancer is one of the most common non-AIDS defining malignancies, especially in men who have sex with men and women living with HIV (WLHIV).
ObjectivesTo evaluate the prevalence and incidence of precursor lesions (high-grade squamous intraepithelial lesions [HSIL]) and anal cancer in our cohort of women and to compare them to cervical lesions; to calculate the percentage of patients that acquire and clear oncogenic genotypes (HR-HPV) in the anal canal; and to determine predictive factors for anal HPV infection.
Patients and methodsProspective-longitudinal study (May 2012–December 2016). At baseline (V1) and follow up visits, anal mucosa samples were taken in liquid medium for cytology and HPV PCR. In cases of abnormal anal cytology and/or positive HR-HPV PCR results, a high resolution anoscopy was performed. Patients were also referred to the gynaecologist.
ResultsNinety five women with an average age of 43.7 years were included. At baseline, 11.6% had cervical abnormalities (4.1% CIN1, 2.2% CIN2/3, 1.1% cervical cancer), 64.3% anal abnormalities (50% LSIL/AIN1, 9.5% HSIL/AIN2/3 and 2.4% anal cancer) and 49.4% had HR-HPV genotypes. During 36 months of follow up, the incidence of anal HSIL was 16×1000 person-years; 14.8% acquired HR-HPV genotypes and 51.2% cleared them, p=0.007. No patients presented CIN1/2/3/ or cervical cancer. In the multivariate analysis we found the following predictive factors for HR-HPV infection: smoking (RR: 1.55, 95% CI: 0.99–2.42), number of sexual partners >3 (RR: 1.69; 95% CI: 1.09–2.62), cervical and anal dysplasia (RR: 1.83; 95% CI: 1.26–2.67) and (RR: 1.55; 95% CI: 1.021–2.35), respectively.
ConclusionsDespite clearance rates of anal oncogenic genotypes being higher than acquisition rates, prevalence and incidence of HSIL were still high and greater than cervical HSIL. Therefore, screening for these lesions should perhaps be offered to all WLHIV.
El cáncer de ano (CA) es uno de los tumores no definitorios de sida más frecuentes, principalmente en hombres que tienen sexo con hombres y mujeres infectadas por VIH.
ObjetivosEvaluar la prevalencia e incidencia de lesiones precursoras (HSIL) y CA en nuestra cohorte de mujeres, y compararlas con las de cérvix; analizar el porcentaje de pacientes que adquieren y aclaran genotipos oncogénicos (VPH-AR) del canal anal; y los factores predictores para dicha infección en ano.
Paciente y métodosEstudio prospectivo-longitudinal (mayo de 2012-diciembre de 2016). En visita basal (V1) y posteriores se tomaban muestras de mucosa anal en medio líquido para citología, y PCR del VPH. En caso de citología anal patológica y/o PCR del VPH-AR positiva se realizaba anoscopia de alta resolución. Además, se enviaban al ginecólogo.
ResultadosNoventa y cinco mujeres de 43,7años fueron incluidas. En V1, el 11,6% tenían patología cervical (4,1% CIN1; 2,2% CIN2/3; 1,1% cáncer de cérvix [CC]); el 64,3% presentaban patología anal (50% LSIL/AIN1, 9,5% HSIL/AIN2/3 y 2,4% CA) y el 49,4%, infección por VPH-AR. Durante 36meses de seguimiento la incidencia de HSIL anal fue de 16×1.000 persona-año; el 14,8% adquirieron VPH-AR y el 51,2% los aclararon, p=0,007; ninguna presentó CIN1/2/3 o CC. En el análisis multivariante encontramos como factores predictores de infección por VPH-AR: tabaquismo (RR: 1,55; IC95%: 0,99-2,42), número de parejas sexuales >3 (RR: 1,69; IC95%: 1,09-2,62), displasia cervical (RR: 1,83; IC95%: 1,26-2,67) y anal (RR: 1,55; IC95%: 1,021-2,35).
ConclusionesA pesar de que el aclaramiento de genotipos oncogénicos en ano era mayor que la adquisición, la prevalencia e incidencia de HSIL era elevada, y superior a la de cérvix, por lo que quizá debería ofertarse el cribado de dichas lesiones a todas las mujeres seropositivas.
At present, anal cancer (AC) is one of the most common non–AIDS-defining tumours in patients with HIV infection,1 especially in men who have sex with men (MSM) and women. HIV-infected women have a far greater incidence than seronegative women.2 AC and cervical cancer (CC) have been closely linked to human papilloma virus (HPV) infection.3 CC is an AIDS-defining neoplasm, and was the most common in seropositive women before high-efficacy antiretroviral therapy (ART) was introduced. After ART was introduced and screening programmes for CC were established, a significant reduction in its incidence occurred.4 The effect that ART could have on the onset of AC has been a matter of debate. Studies conducted before it was administered or close to when it was introduced found an increased incidence which might have been linked to increased patient survival.5–8 However, more recent studies conducted in seropositive MSM have found that antiretroviral therapy could have a certain protective effect in the presence of a high-grade intraepithelial lesion or precursor lesion (HSIL) and AC.9–11
For now, there is no consensus or consistency among the various HIV scientific associations with respect to recommendations on screening for HSIL and AC—not only in terms of procedure and technique, but also in terms of type of patient to be screened.12,13 In Spain, the 2017 GESIDA consensus document on diagnosis and treatment of sexually transmitted infections in adults, children and adolescents includes performing anal cytology in HIV-positive patients with condylomas, MSM and women with cervical dysplasia.12 Cytology is the most commonly used technique to screen for HSIL and CA. However, the sensitivity of this tool is variable and limited. This means that a non-negligible percentage of patients with these lesions are underdiagnosed, as some studies have shown.14–16
With this study we sought to evaluate the prevalence and incidence of HSIL and AC in our cohort of seropositive women (HIV+W), and to compare them with women with CC. We also sought to analyse the percentage of women who acquire and clear infection with oncogenic genotypes of HPV in the anal canal and the predictive factors for the appearance of these genotypes in the anus.
Patients and methodsDesign: a single-centre, longitudinal, prospective study consisting of HIV+W who were consecutively enrolled, after signing the informed consent form, in a programme for screening, diagnosis, treatment and follow-up of dysplastic lesions of the anal mucosa (May 2012–December 2016). The patients were recruited from the infectious disease unit at a Spanish tertiary hospital. The study was evaluated and approved by the independent ethics committee of the centre where the patients were recruited. Inclusion criteria: >18 years of age, HIV+W. Exclusion criteria: men with HIV infection and a history of anal canal neoplasm.
At the visit, the patients were informed of the study conditions and objectives and provided with an informed consent form to sign. Their epidemiological, clinical and laboratory data were collected and processed according to the current Spanish data protection legislation (Organic Law 15/1999, of 13 December, on Personal Data Protection).
At the baseline visit (V1) and subsequent visits (Vx), the following were collected:
- -
Clinical/epidemiological variables: age, history of perianal/genital condylomas and cervical disease (CIN 1/2/3 and CC), sex and number of sexual partners in the last 12 months and throughout life, anal sex, use of a condom, smoking, alcohol use (standard drink units [SDUs]), parenteral drug addiction (PDA), nationality, level of education, months since HIV diagnosis, stage of HIV according to CDC classification; months in antiretroviral treatment (ART), virologic failure (defined as viral RNA >50copies/ml in at least two determinations in the past 6 months) and use of concomitant treatment. Other infections: chronic liver disease due to hepatitis B virus (HBV) or hepatitis C virus (HCV), positive serology for syphilis and other sexually transmitted diseases, and latent tuberculosis infection (treated or active).
- -
The laboratory variables included were nadir CD4 lymphocyte count; CD4 count and viral load at the time of HIV diagnosis; and CD4 count, CD8 count and viral load at the time of enrolment in the study.
At V1 and Vx, 2 samples were taken from the mucosa of the anal canal with a pair of cotton swabs soaked in normal saline: one for HPV detection and genotyping through a qualitative polymerase chain reaction (PCR) (Linear Array HPV Genotyping Test), performed with the GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Roche, Switzerland), and one for cytology. Both samples were immersed in a liquid medium (thin-layer liquid). The thin-layer technique was used for the cytology study (ThinPrep 2000 processor, Hologic). Both samples were sent to the pathology laboratory, where a single pathologist (JE) provided opinions on cytologies and validated HPV PCR results. Genotypes 16, 18, 26, 31, 33, 35, 39, 45, 51–53, 56, 58, 59, 66, 68, 73 and 82 were considered high-risk (HR-HPV). Genotypes 6, 11, 34, 40, 42–44, 54, 55, 57, 61, 70–72, 81, 83, 84 and 89 were considered low-risk (LR-HPV). Viruses 39, 45, 59 and 68 were classified as subspecies of HPV genotype 18, and genotypes 31, 33, 35, 52, 58 and 67 were classified as subspecies of HPV genotype 16.17
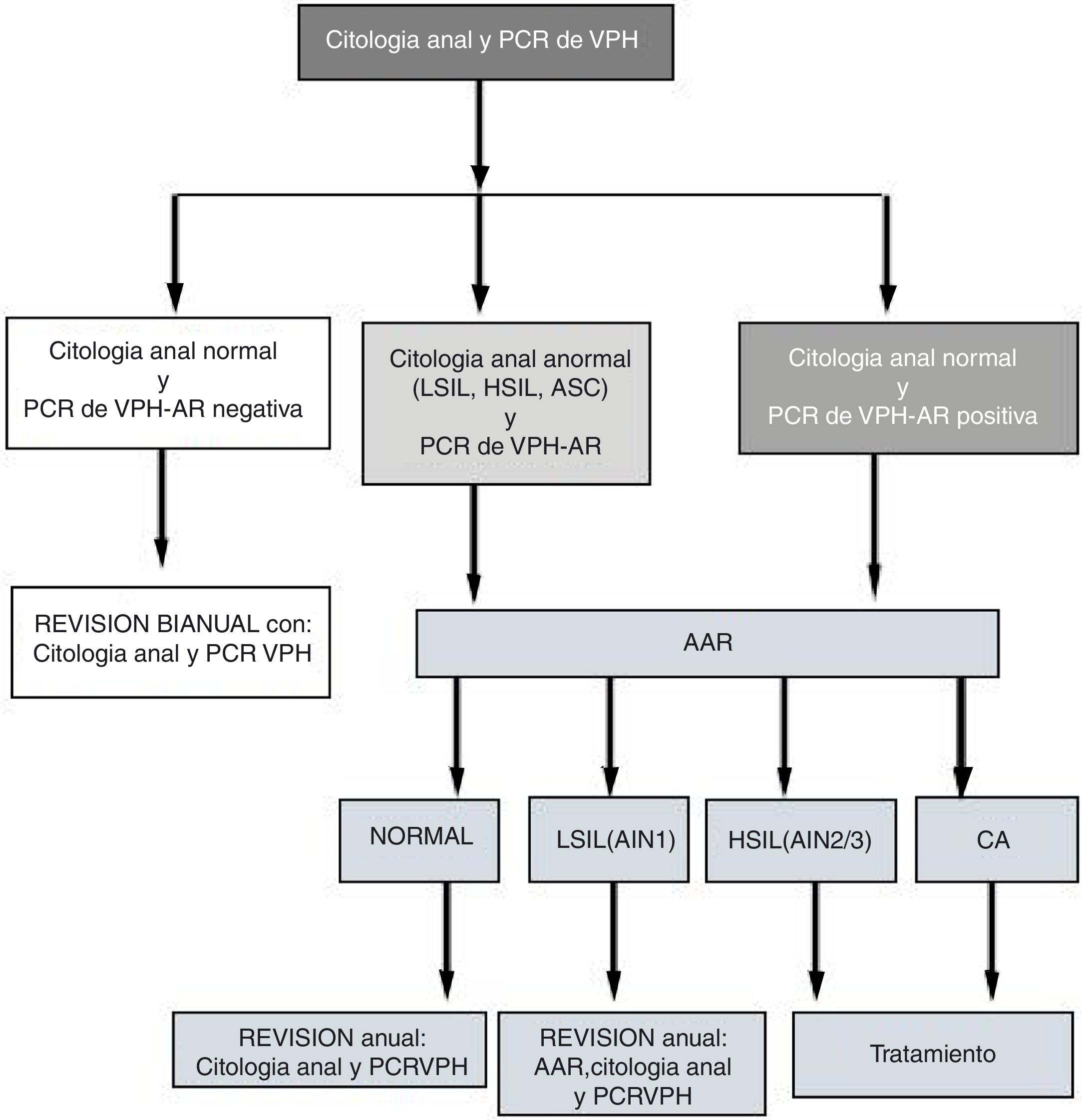
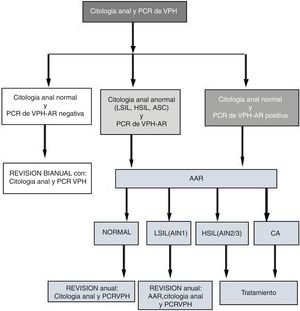
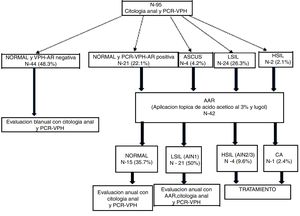
At the visits (V1 and Vx), patients with abnormal cytology and/or positive high-risk (HR) HPV PCR underwent high-resolution anoscopy (HRA). Patients with normal HRA and negative HR-HPV PCR were evaluated every two years with another cytology and PCR. Patients with positive HPV PCR and/or HRA with LSIL (AIN 1) were evaluated every year. Those diagnosed with HSIL or AC were treated (Fig. 1). We developed the follow-up algorithm (Fig. 1) based on our own data on sensitivity, specificity and positive and negative predictive values for anal cytology and high-risk HPV PCR to predict HSIL.14
HRA procedures were performed with the Carl Zeiss 150 FC© colposcope (Carl Zeiss, Oberkochen, Germany) 4–12 weeks after anal cytology was done. Prior to HRA, the patient underwent an inspection and a digital rectal examination. After this, a transparent disposable anoscope was inserted, 5ml of acetic acid were instilled through the anoscope and the acetic acid was left in place for around 3min. Next, the anoscope was removed and the mucosa was inspected. After that, Lugol's 5% solution was instilled and left in place for a minute, and the mucosa was inspected again. Samples were taken both from apparently normal mucosa in the 4 quadrants and from areas suggestive of dysplasia (aceto-white/Lugol's-negative). Biopsies were performed with an endoscopic retrograde cholangiopancreatography (ERCP) catheter.
We used the Bethesda classification for anal and cervical cytology.18 This classification divides lesions into 4 types: atypical squamous cells (ASC), atypical squamous cells-high (ASC-H) in which high grade cells cannot be ruled out, low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL).
The histology classification used for both the anus and the cervix was the “Squamous Terminology (LAST) Standardisation Project for HPV” consensus classification. This classifies anal lesions as LSIL (AIN 1/condyloma), HSIL (AIN 2/AIN 3) or invasive anal canal carcinoma (AC), and cervical lesions as CIN 1, CIN 2, CIN 3 or CC. P16 was used according to the recommendations of the LAST consensus.19
Cytology and histology studies were always performed by the same pathologist (JE).
At V1 and Vx, the women were referred to a gynaecologist for cervical cytology (the same liquid medium as for anal cytology was used). If it was abnormal, the women underwent colposcopy with acetic acid and Lugol's staining, after which only aceto-white Lugol's-negative areas were biopsied. The samples were sent to the same pathology department.
Definition of variables:
- -
Abnormal cytology: cytology featuring ASCUS, ASCUS-H, LSIL or HSIL.
- -
Histology with HSIL+ anal lesion: histology featuring anything from HSIL (AIN 2 or AIN 3) to invasive cancer.
- -
Histology with LSIL+ anal lesion: histology featuring anything from LSIL (condyloma/AIN 1) to invasive cancer.
- -
Clearance of HR-HPV infection: when a patient having had infection in the anal mucosa with any HR-HPV genotype at the baseline visit (V1) was not found to have any HR-HPV genotype at the last visit.
- -
Acquisition of HR-HPV infection: when a patient presented a new HR-HPV genotype in the anal mucosa at the last visit that had not been present at the baseline visit (V1).
Sample size. We enrolled 95 out of a total of 149 women who received care on the outpatient unit of an infectious disease department belonging to a Spanish tertiary hospital. These women voluntarily agreed to take part in the programme.
Descriptive analysis. We prepared a description of the main variables collected in the study, calculated measures of central tendency and dispersion (mean, standard deviation, median and percentiles) for the quantitative variables, and calculated absolute frequencies with a 95% confidence interval for the qualitative variables. HPV prevalence as well as anal mucosa cytology and histology findings were calculated with a 95% confidence interval.
Bivariate analysis. This was used to examine the relationship between potential risk factors and HR-HPV infection. Student's t test for independent samples was used for the quantitative variables that followed a normal distribution. The Mann–Whitney U test was used for those that did not follow such a distribution. Pearson's chi-squared test was used for the qualitative variables that met the application criteria, and Fisher's exact test was used for the ones that did not. The Kolmogorov–Smirnov test was used to confirm whether the different variables fulfilled the hypothesis of normality. A level of significance of 0.05 was applied to all tests. The statistical software programme used was SPSS 20.0.
Multivariate analysis. This was performed by means of Poisson regression using the Stata Release 12 statistical software programme with robust error variance and calculating relative risk (RR) as well as its 95% confidence interval. As we performed this type of regression based on the high prevalence of anal infection with HR-HPV in our cohort of HIV-infected women, calculating the RR proved more suitable than calculating the odds ratio. The method of selection of variables was by successive steps backwards, considering a p<0.05 to be an entry criterion and a p>0.10 to be an exit criterion. The model included both variables that proved statistically significant in the bivariate analysis and clinically significant variables. It ultimately consisted of the following variables: more than 3 sexual partners throughout life, smoking and abnormal anal and cervical cytology.
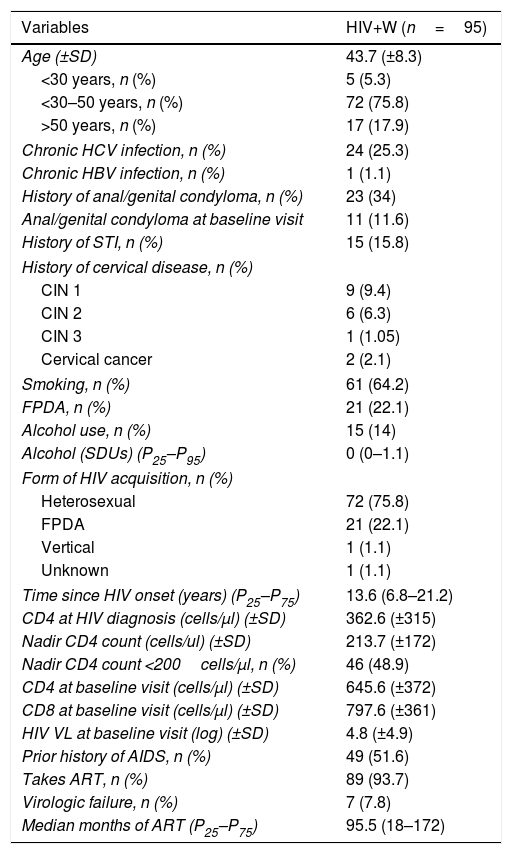
ResultsBaseline characteristics of the patients enrolledWe enrolled 95 women with a mean age of 43.7 years (75.8% were 30–50 years of age). Their median time since HIV onset was 13.6 years (P25–P75: 6.8–21.2), their nadir CD4 count was 213.7cells/μl, 93.6% had been on antiretroviral treatment for 10 years (P25–P75: 5–16.8), their mean CD4 count was 648.7cells/μl (CD4/CD8: 0.95) and 7.8% of them were in virologic failure. All other variables are found in Table 1.
Baseline characteristics of the cohort.
| Variables | HIV+W (n=95) |
|---|---|
| Age (±SD) | 43.7 (±8.3) |
| <30 years, n (%) | 5 (5.3) |
| <30–50 years, n (%) | 72 (75.8) |
| >50 years, n (%) | 17 (17.9) |
| Chronic HCV infection, n (%) | 24 (25.3) |
| Chronic HBV infection, n (%) | 1 (1.1) |
| History of anal/genital condyloma, n (%) | 23 (34) |
| Anal/genital condyloma at baseline visit | 11 (11.6) |
| History of STI, n (%) | 15 (15.8) |
| History of cervical disease, n (%) | |
| CIN 1 | 9 (9.4) |
| CIN 2 | 6 (6.3) |
| CIN 3 | 1 (1.05) |
| Cervical cancer | 2 (2.1) |
| Smoking, n (%) | 61 (64.2) |
| FPDA, n (%) | 21 (22.1) |
| Alcohol use, n (%) | 15 (14) |
| Alcohol (SDUs) (P25–P95) | 0 (0–1.1) |
| Form of HIV acquisition, n (%) | |
| Heterosexual | 72 (75.8) |
| FPDA | 21 (22.1) |
| Vertical | 1 (1.1) |
| Unknown | 1 (1.1) |
| Time since HIV onset (years) (P25–P75) | 13.6 (6.8–21.2) |
| CD4 at HIV diagnosis (cells/μl) (±SD) | 362.6 (±315) |
| Nadir CD4 count (cells/ul) (±SD) | 213.7 (±172) |
| Nadir CD4 count <200cells/μl, n (%) | 46 (48.9) |
| CD4 at baseline visit (cells/μl) (±SD) | 645.6 (±372) |
| CD8 at baseline visit (cells/μl) (±SD) | 797.6 (±361) |
| HIV VL at baseline visit (log) (±SD) | 4.8 (±4.9) |
| Prior history of AIDS, n (%) | 49 (51.6) |
| Takes ART, n (%) | 89 (93.7) |
| Virologic failure, n (%) | 7 (7.8) |
| Median months of ART (P25–P75) | 95.5 (18–172) |
VL: HIV viral load; FPDA: former parenteral drug addiction; STI: sexually transmitted infection; HIV+W: HIV-positive women; HBV: chronic hepatitis B infection; HCV: chronic hepatitis C infection; SDU: standard drink unit.
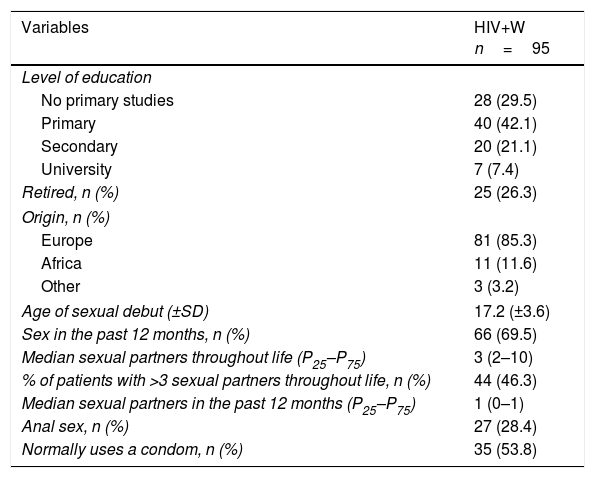
Of these women, 29.5% had not undergone any type of study, 46.3% had had more than 3 sexual partners throughout life and 28.4% had had anal sex. In addition, 64.2% were smokers, 11.6% had genital/perianal warts and 18.9% had a history of cervical disease (9.5% CIN 1, 6.3% CIN 2, 1.05% CIN 3 and 2.1% CC). All other variables are presented in Table 2.
Demographic characteristics and characteristics related to sexual habits.
| Variables | HIV+W n=95 |
|---|---|
| Level of education | |
| No primary studies | 28 (29.5) |
| Primary | 40 (42.1) |
| Secondary | 20 (21.1) |
| University | 7 (7.4) |
| Retired, n (%) | 25 (26.3) |
| Origin, n (%) | |
| Europe | 81 (85.3) |
| Africa | 11 (11.6) |
| Other | 3 (3.2) |
| Age of sexual debut (±SD) | 17.2 (±3.6) |
| Sex in the past 12 months, n (%) | 66 (69.5) |
| Median sexual partners throughout life (P25–P75) | 3 (2–10) |
| % of patients with >3 sexual partners throughout life, n (%) | 44 (46.3) |
| Median sexual partners in the past 12 months (P25–P75) | 1 (0–1) |
| Anal sex, n (%) | 27 (28.4) |
| Normally uses a condom, n (%) | 35 (53.8) |
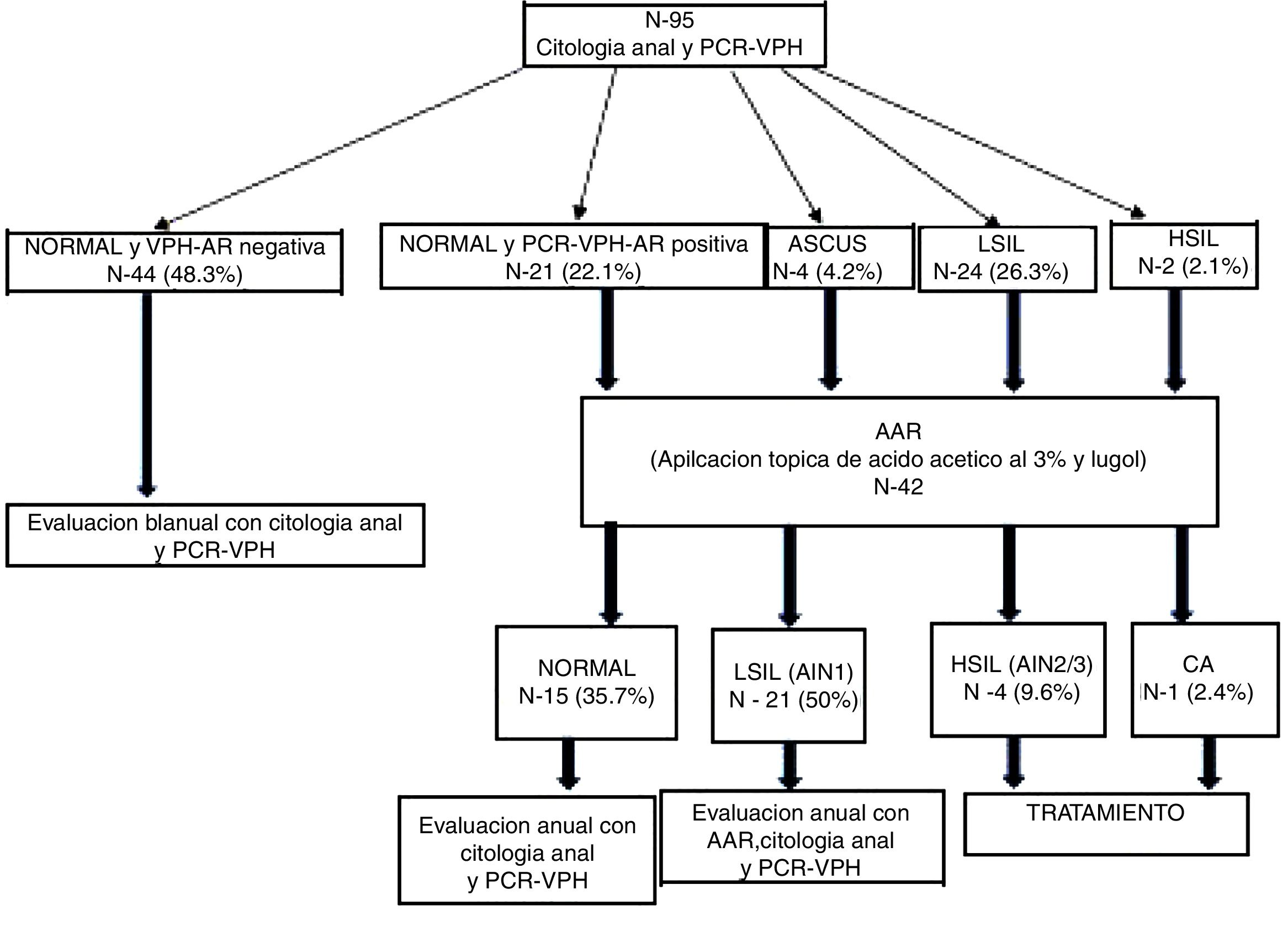
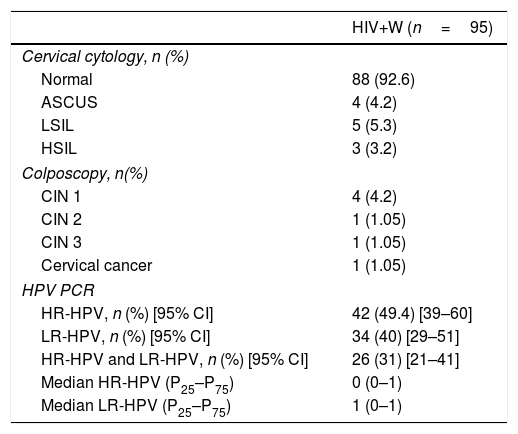
Of the HIV+W, 92.6% had a normal cervical cytology, 4.2% had ASCUS, 5.2% had LSIL and 3.2% had HSIL (Table 3). Regarding anal cytology results, we found that 68.4% of patients had normal cytology, 25.3% had LSIL, 2.1% had HSIL and 4.2% had ASCUS (Fig. 2), with statistically significant differences between the two types of cytology (p=0.0001).
Results of cervical cytology, colposcopy and HPV PCR of the anal mucosa.
| HIV+W (n=95) | |
|---|---|
| Cervical cytology, n (%) | |
| Normal | 88 (92.6) |
| ASCUS | 4 (4.2) |
| LSIL | 5 (5.3) |
| HSIL | 3 (3.2) |
| Colposcopy, n(%) | |
| CIN 1 | 4 (4.2) |
| CIN 2 | 1 (1.05) |
| CIN 3 | 1 (1.05) |
| Cervical cancer | 1 (1.05) |
| HPV PCR | |
| HR-HPV, n (%) [95% CI] | 42 (49.4) [39–60] |
| LR-HPV, n (%) [95% CI] | 34 (40) [29–51] |
| HR-HPV and LR-HPV, n (%) [95% CI] | 26 (31) [21–41] |
| Median HR-HPV (P25–P75) | 0 (0–1) |
| Median LR-HPV (P25–P75) | 1 (0–1) |
CIN: cervical intraepithelial neoplasia; HPV: human papilloma virus; HR-HPV: high-risk HPV, LR-HPV: low-risk HPV.
Flow of patients from anal cytology and HR-HPV PCR to HRA.
HRA: high-resolution anoscopy; AIN: anal intraepithelial neoplasm; ASCUS: atypical squamous cells of undetermined significance; AC: anal cancer; HSIL: high-grade intraepithelial lesion; LSIL: low-grade intraepithelial lesion; HPV PCR: human papilloma virus protein chain reaction.
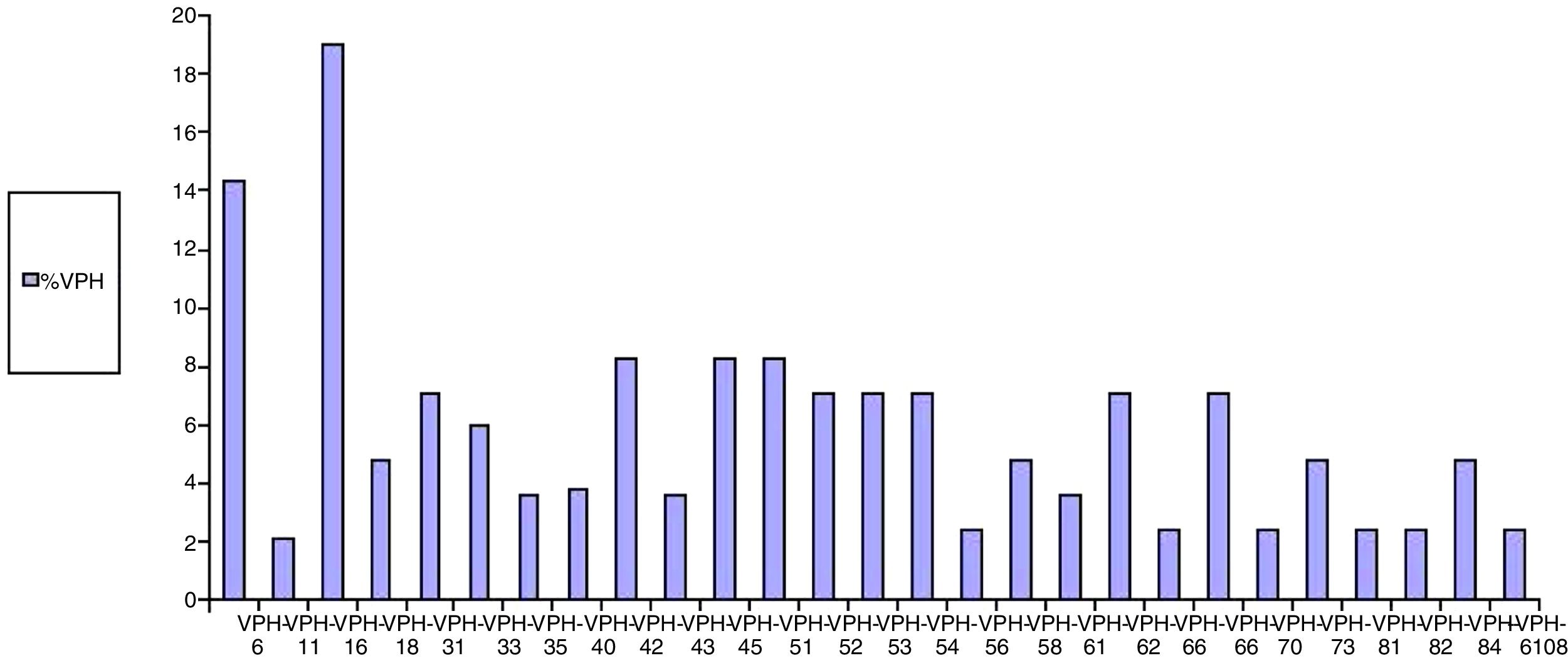
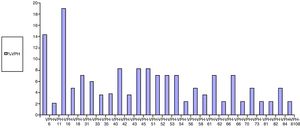
Regarding the results of anal HPV PCR, 49.4% of women (95% CI: 39–60) had infection with high-risk genotypes, with a median number of oncogenic genotypes per patient of 0 (P25–P75: 0–1); 40% (95% CI: 29–51) had infection with low-risk genotypes, with a median of 0 (P25–P75: 0–1), and 31% had simultaneous infection with low- and high-risk genotypes (95% CI: 21–41) (Table 3). The HPV genotypes most commonly isolated in the anal mucosa were as follows: among low-risk genotypes, 6 (14.3%), 42 (8.3%), 62 (7.1%), 73 (4.8%) and 84 (4.8%), and, among high-risk genotypes, 16 (19%), 45 (8.3%), 51 (8.3%) and 55 (8.3%) (Fig. 3).
Of the 42 HRA procedures performed, 38.1% were normal, 50% showed LSIL (AIN 1), 9.5% showed HSIL (AIN 2/3) and 2.4% showed AC (Fig. 2). Of the 12 colposcopies performed, 5.2% were normal, 4.2% were CIN 1, 1.05% were CIN 2, 1.05% were CIN 3 and 1.05% were CC.
Results of follow-upDuring the median 36 months (P25–75: 24.8–47.3) of follow-up, three women developed new cases of anal HSIL, whose incidence was 16×1000 people per year. Of them, 14.8% acquired HR-HPV and 51.2% cleared it; the differences were statistically significant (p=0.007).
Two women died of their disease. One died of anal cancer 24 months after being diagnosed at the baseline visit and undergoing radiotherapy, chemotherapy and abdominopelvic amputation. The other died of a recurrence of Hodgkin lymphoma.
Of the 8 women with precursor lesions of AC (HSIL) in the cohort, one of them refused to receive treatment; at her 12-month check-up, the lesion (AIN 2) had disappeared and she had LSIL (AIN 1). Another received intra-anal imiquimod 3 times weekly for 16 weeks. Her disease not only did not respond, but progressed, with greater impairment of quadrants 1–3. As a result she required electrocauterization/fulguration of her lesions with a scalpel. The remaining 6 women received electrocauterization/fulguration. All 100% of the patients treated did not present precursor lesions or AC again during a median follow-up period of 30.5 months (P25–P75: 17.5–36.5).
None of the women presented CIN 1, CIN 2, CIN 3 or CC during follow-up. However, 1.05% presented vulvar intraepithelial neoplasia grade 1 (VIN 1) and 1.05% presented VIN 3.
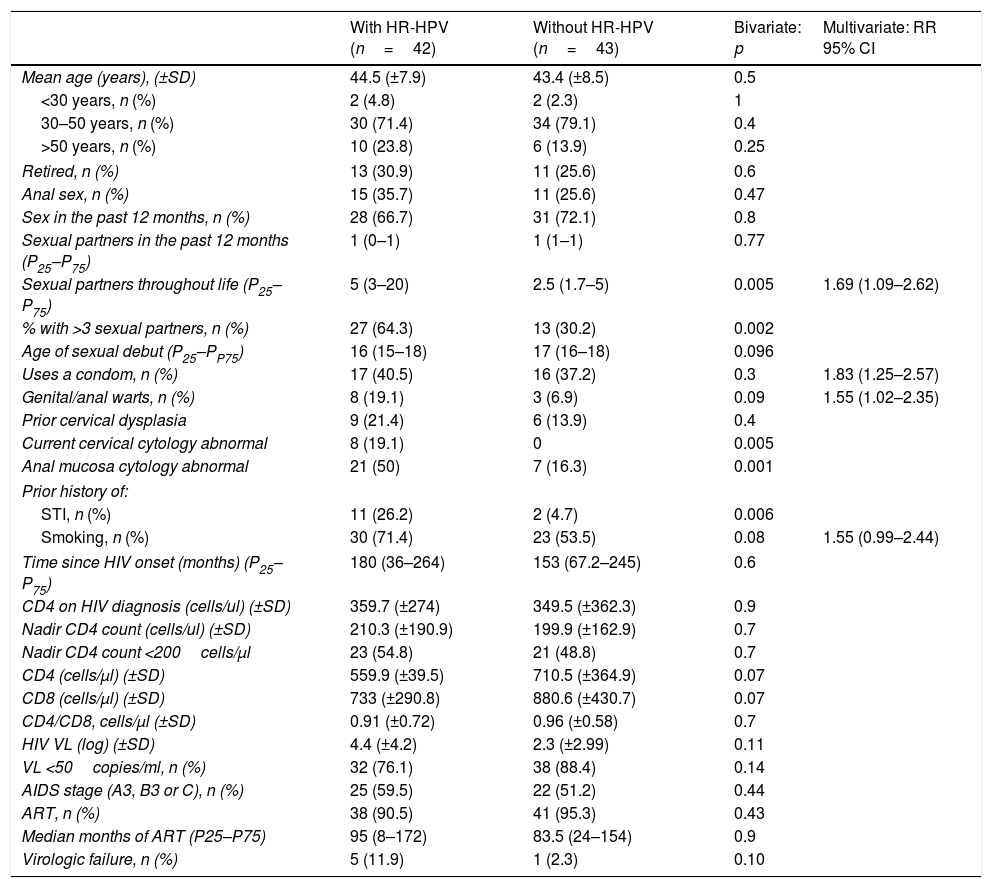
Factors that predict HR-HPV infectionThe bivariate analysis found the following to be risk factors for anal infection with HR-HPV: history of sexually transmitted infection (STI) (26.2% vs. 4.7%; p=0.006), cervical dysplasia (19.1% vs. 0%; p=0.005), abnormal anal cytology (50% vs. 16.3%; p=0.001) and more than 3 sexual partners throughout life (64.3% vs. 30.2%; p=0.002). All other variables may be seen in Table 4.
Risk factors for HR-HPV infection in the anal mucosa of HIV+W. Bivariate and multivariate analysis.
| With HR-HPV (n=42) | Without HR-HPV (n=43) | Bivariate: p | Multivariate: RR 95% CI | |
|---|---|---|---|---|
| Mean age (years), (±SD) | 44.5 (±7.9) | 43.4 (±8.5) | 0.5 | |
| <30 years, n (%) | 2 (4.8) | 2 (2.3) | 1 | |
| 30–50 years, n (%) | 30 (71.4) | 34 (79.1) | 0.4 | |
| >50 years, n (%) | 10 (23.8) | 6 (13.9) | 0.25 | |
| Retired, n (%) | 13 (30.9) | 11 (25.6) | 0.6 | |
| Anal sex, n (%) | 15 (35.7) | 11 (25.6) | 0.47 | |
| Sex in the past 12 months, n (%) | 28 (66.7) | 31 (72.1) | 0.8 | |
| Sexual partners in the past 12 months (P25–P75) | 1 (0–1) | 1 (1–1) | 0.77 | |
| Sexual partners throughout life (P25–P75) | 5 (3–20) | 2.5 (1.7–5) | 0.005 | 1.69 (1.09–2.62) |
| % with >3 sexual partners, n (%) | 27 (64.3) | 13 (30.2) | 0.002 | |
| Age of sexual debut (P25–PP75) | 16 (15–18) | 17 (16–18) | 0.096 | |
| Uses a condom, n (%) | 17 (40.5) | 16 (37.2) | 0.3 | 1.83 (1.25–2.57) |
| Genital/anal warts, n (%) | 8 (19.1) | 3 (6.9) | 0.09 | 1.55 (1.02–2.35) |
| Prior cervical dysplasia | 9 (21.4) | 6 (13.9) | 0.4 | |
| Current cervical cytology abnormal | 8 (19.1) | 0 | 0.005 | |
| Anal mucosa cytology abnormal | 21 (50) | 7 (16.3) | 0.001 | |
| Prior history of: | ||||
| STI, n (%) | 11 (26.2) | 2 (4.7) | 0.006 | |
| Smoking, n (%) | 30 (71.4) | 23 (53.5) | 0.08 | 1.55 (0.99–2.44) |
| Time since HIV onset (months) (P25–P75) | 180 (36–264) | 153 (67.2–245) | 0.6 | |
| CD4 on HIV diagnosis (cells/ul) (±SD) | 359.7 (±274) | 349.5 (±362.3) | 0.9 | |
| Nadir CD4 count (cells/ul) (±SD) | 210.3 (±190.9) | 199.9 (±162.9) | 0.7 | |
| Nadir CD4 count <200cells/μl | 23 (54.8) | 21 (48.8) | 0.7 | |
| CD4 (cells/μl) (±SD) | 559.9 (±39.5) | 710.5 (±364.9) | 0.07 | |
| CD8 (cells/μl) (±SD) | 733 (±290.8) | 880.6 (±430.7) | 0.07 | |
| CD4/CD8, cells/μl (±SD) | 0.91 (±0.72) | 0.96 (±0.58) | 0.7 | |
| HIV VL (log) (±SD) | 4.4 (±4.2) | 2.3 (±2.99) | 0.11 | |
| VL <50copies/ml, n (%) | 32 (76.1) | 38 (88.4) | 0.14 | |
| AIDS stage (A3, B3 or C), n (%) | 25 (59.5) | 22 (51.2) | 0.44 | |
| ART, n (%) | 38 (90.5) | 41 (95.3) | 0.43 | |
| Median months of ART (P25–P75) | 95 (8–172) | 83.5 (24–154) | 0.9 | |
| Virologic failure, n (%) | 5 (11.9) | 1 (2.3) | 0.10 | |
VL: viral load; STI: sexually transmitted infection; RR: relative risk; HPV: human papilloma virus.
Finally, the multivariate analysis found the following to be risk factors for anal infection with oncogenic genotypes: smoking (RR: 1.55; 95% CI: 0.99–2.42), more than 3 sexual partners throughout life (RR: 1.69; 95% CI: 1.09–2.62), cervical dysplasia (RR: 1.83; 95% CI: 1.26–2.67) and anal dysplasia (RR: 1.55; 95% CI: 1.021–2.35) (Table 4).
DiscussionOne out of every 10 HIV-positive women in our cohort had a precursor lesion of anal cancer associated with HPV, with an incidence of 16×1000 people per year, and up to 50% had a low-grade lesion (LSIL/AIN 1). A comparative study of seropositive versus seronegative women found a rate of HSIL in HIV-infected women similar to ours (9%)20; however, it found a much lower prevalence of LSIL (just 12%). The difference between the results of that study and those of our study could be attributed to the fact that, in that study, anoscopy was performed only in case of abnormal cytology. In our study, based on our own data on the sensitivity of cytology and HPV PCR in screening for HSIL,14 anoscopy was performed both in women with dysplastic cytology and in women with a positive PCR for any oncogenic genotype of HPV. In another study in HIV+W alone, the rate of HSIL+ was 12%, and the main risk factors for this type of lesion were found to be cervical disease and anal infection with genotype 16.21 However, in a retrospective study consisting of 745 HIV+W, there was a much higher prevalence of HSIL (26%), which was associated with smoking and abnormal cervical cytology.22 Finally, a systematic review on precursor lesions of anal cancer associated with HPV in HIV+W showed variability in prevalence figures in these lesions on comparison of different cohorts (from 3% to 26%).2 When we compared the prevalence in our cohort of anal HSIL+ to cervical HSIL+, we found a higher rate of dysplasia in the anus, with statistically significant differences. Moreover, for the median follow-up period of 3 years, we found no cases of a cervical precursor lesion or cervical cancer. These results were consistent with those reported to date in the literature, in which the incidence of CC has been gradually reducing in cohorts of HIV-infected women thanks in part to the introduction of antiretroviral treatment.23
Regarding anal cancer, the prevalence that we discovered in our cohort was much the same as that of CC (lower than 3% in both cases). In the median 36 months of follow-up, none of the women presented anogenital cancer again, and those who were diagnosed with and treated for a precursor lesion associated with HPV also did not experience progression to AC. One of them, who had anal HSIL, even experienced spontaneous remission. This could be due, among other reasons, to the fact that more than 90% were undergoing antiretroviral treatment and had an excellent viro-immunological status. A meta-analysis in seropositive patients on anal infection with HPV and neoplastic lesions associated with this infection found that anal intraepithelial neoplasia progressed to anal cancer at a lower rate than CIN 3 progressed to cervical cancer (1 in 600× year in men who have sex with men who are seropositive vs. 1 in 80× year in women).24
AC is a non-AIDS-defining disease that is highly prevalent in the HIV-positive population versus the general population.25 A variable incidence from 3.9 to 30×100,000 people per year has been reported in HIV-infected women. These figures are as high as 63.8 cases per 100,000 people per year in those with CC or CIN 3.2 Recent publications on cohorts of HIV-infected patients who were men who had sex with men have reported a protective effect of ART against the development of precursor lesions and AC associated with HPV9–11,26,27; this could account for our findings.
Regarding the prevalence of HPV infection of the anal mucosa in the women we studied, we found that as many as half of them were infected with high-risk genotypes, 2/5 were infected with low-risk genotypes and 1/3 were infected with both genotypes. HPV genotype 16 was the most commonly isolated genotype in the anal mucosa, having been isolated in nearly one out of every five patients. Genotype 6 was the most commonly isolated low-risk genotype. A recently published study on HPV prevalence in HIV+W showed that approximately half of patients were infected with oncogenic genotypes, the most common one being 16; these data are very similar to our study's data.28 The SUN prospective cohort study, consisting of 120 HIV+W, found a prevalence of 85% of these genotypes in the anus. That study may have differed from our data and prior data28 in part because its cohort, compared to ours, had a lower percentage of patients in antiretroviral treatment (ART), in virologic suppression and with CD4 counts >500cells/ml (just 77%, 73% and 36%, respectively).29
We also found that one out of every 7 women acquired high-risk genotypes, and although more than half cleared them during follow-up, the incidence of anal HSIL in our cohort was high. A retrospective study by the AIDS Clinical Trials Group (ACTG) (A5029) showed that HIV+W on antiretroviral treatment with a CD4 count >350cells/μl had a higher rate of clearance and, in the case of an HIV viral load >400copies/ml, a higher rate of acquisition.30 These data could account for our results, since 93.6% of our patients received ART, our patients had a mean CD4 count of 648.7cells/μl and just 7.8% were found to be in virologic failure.
When we analysed potential predictive factors for infection of the anal mucosa with oncogenic genotypes, we discovered that those with a statistically significant link were smoking, more than three sexual partners throughout life, cervical dysplasia and anal dysplasia. These data were consistent with those reported in 2016 by the ANRS-C017 VIHGY study group, who found the following to be factors associated with anal infection with oncogenic viruses in HIV+W: dysplastic cervical cytology, cervical infection with high-risk HPV and CD4 <350cells/ml. This cohort had rates of ART and CD4 >500cells/ml close to 100%, and more than 80% of patients were found to be in virologic suppression.28 Smoking in HIV-positive patients has also been previously linked to dysplasia of the anal canal,31 as well as infection with a high-risk virus in the cervix and CIN 2+ lesions in HIV+W.32 Regarding sexual habits, both the type of relationship and a higher number of sexual partners have been considered to be classic factors in infection with oncogenic HPV in the anal mucosa.33
One of the main limitations of our study lay in its single-centre design. Other limitations derived from the fact that we did not have access to PCR for HPV from the cervix (due to problems beyond the control of the researchers and related to budget constraints), and still others were related to the exclusion criteria. The strengths of the study included its longitudinal and prospective design; the fact that the patients always received care from the same clinic which was responsible for taking samples as well as HRA; and the fact that cytology and histology were interpreted by the same pathologist.
In conclusion, in this late period of antiretroviral therapy, the prevalence and incidence of precursor lesions for anal cancer associated with HPV in our cohort of HIV+W were higher than those for cervical cancer, despite the fact that clearance of oncogenic genotypes in the anal mucosa exceeded acquisition. Therefore, we believe that screening for these lesions should be offered to all HIV-infected women, with special emphasis on (in no particular order) those who smoke, those who have a higher number of sexual partners and those with anogenital dysplasia.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hidalgo-Tenorio C, de Jesus SE, Esquivias J, Pasquau J. Alta prevalencia e incidencia de lesiones precursoras de cáncer anal asociada a la infección por VPH en mujeres VIH positivas en la era tardía del TAR. Enferm Infecc Microbiol Clin. 2018;36:555–562.