Treatment of gonorrhoea is threatened by antimicrobial resistance, and decreased susceptibility to recommended therapies is emerging. Thus, gonococcal infection (GI) is becoming a public health problem. The objectives of the present study were to monitor the antimicrobial sensitivity in Neisseria gonorrhoeae (NG) during 2011–2015 and to study their genogroups.
MethodsAntimicrobial susceptibility was studied by disc diffusion, in addition to the agar dilution method for cefixime and ceftriaxone and the Etest® for azithromycin. Genotyping was performed by the NG multi-antigen sequence typing (NG-MAST) method. Genogroups of closely related sequence types (STs) were defined.
ResultsAll the strains were susceptible to cefixime, ceftriaxone and gentamicin and 1.8% of the strains were resistant to azithromycin. A total of 531 STs and 6 genotypes (Gs) were identified during 2012–2015 period. G2992 was the largest and was associated with resistance to azithromycin, and with men who have sex with men (MSM), alongside G2400. G1407 and G2400 strains were related to high minimum inhibitory concentration (MICs) to cefixime and G1407 also to ceftriaxone. For the first time, G1861 and G2018 were described and associated with ciprofloxacin resistance and G2018 also with high MICs to ceftriaxone.
ConclusionMolecular typing is a useful tool to predict antimicrobial resistance. These results show the need to develop novel antimicrobials or to design new antimicrobial therapies based on drugs that show their efficacy against GI. This also highlights the importance of developing sexually transmitted infection (STI) surveillance in homosexual populations.
el tratamiento de la gonorrea está amenazado por la resistencia antimicrobiana, y la disminución de la sensibilidad a las terapias recomendadas está emergiendo. Por ello la infección gonocócica (IG) se está convirtiendo en un problema de salud pública.
Métodosla sensibilidad antimicrobiana se estudió por el método de difusión en disco, cefixima y ceftriaxona fueron testados por el método de dilución en agar y azitromicina por Etest. El genotipado se realizó por el método NG multi-antigen sequence typing (NG-MAST). Se definieron genogrupos con secuenciotipos (STs) relacionados.
Resultadostodas las cepas fueron sensibles a cefixima, ceftriaxona y gentamicina y el 1,8% resistentes a azitromicina. Se identificaron 531 STs y 6 genotipos (Gs) durante el período 2012-2015. El G2992 fue el más grande y se relacionó con resistencia a azitromicina, y con hombres que tienen sexo con hombres (HSH) junto con el G2400. Las cepas pertenecientes a los G1407 y G2400 se relacionaron con altas concentraciones mínimas inhibitorias (CMIs) a cefixima y el G1407 también a ceftriaxona. Se describe por primera vez la presencia del G1861 y G2018 y su relación con la resistencia a ciprofloxacino y la relación del G2018 con alta CMI a ceftriaxona.
ConclusiónEl tipado molecular es una herramienta útil para predecir la resistencia antimicrobiana. Estos resultados muestran la necesidad de desarrollar nuevos antimicrobianos o nuevas terapias basadas en fármacos que demuestren su eficacia contra la IG. También muestra la importancia del desarrollo de la vigilancia de las infecciones de transmisión sexual (ITS) en la población homosexual.
Gonococcal infection (GI) is the second most prevalent sexually transmitted infection (STI) of bacterial origin, followed by Chlamydia trachomatis, and represents an important public health problem due to its prevalence as well as to the complications it can cause. In 2016, 75,349 cases of GI were reported in 27 countries of the European Union and the European Economic Area (EU/EEA) had an incidence of 18.8 cases per 100,000 inhabitants.1 Although GIs occasionally are asymptomatic, especially in women, they can cause severe complications such as epididymitis, pelvic inflammatory disease, ectopic pregnancy and an increased transmission risk of the human immunodeficiency virus (HIV).2 Due to these reasons, an appropriate diagnosis and an effective treatment are necessary. However Neisseria gonorrhoeae (NG) has developed resistance mechanisms to all the antimicrobial introduced for its treatment.3
At present, the “Centers for Disease Control and Prevention” (CDC) and the World Health Organisation (WHO) recommend a dual therapy of 250mg of ceftriaxone along with 1g of azithromycin, while the combination of 400mg cefixime and 1g azithromycin constitutes the alternative therapy.4,5 On the other hand, some clinical guidelines recommend only treatment with ceftriaxone 1g as a single dose.6 However, several countries have reported cases of gonococcal strains with resistance or diminished susceptibility to ESCs and treatment failures with cefixime and ceftriaxone.7–9 Besides, resistance to azithromycin has increased worldwide. Due to these facts, epidemiological surveillance programmes have been developed to detect patterns of antimicrobial resistance at regional, national and international levels, in order to design effective treatments against GI.
Molecular epidemiological surveillance is a useful tool to identify correlations between NG genotypes and antimicrobial resistance profiles, in order to have a better understanding of the emergence and propagation of resistant gonococcal strains.
The objectives of this study are to analyse the antimicrobial susceptibility of the NG strains isolated from patients belonging to the Integrated Health Organisation (OSI) Bilbao-Basurto (Spain), and to perform their genotyping with the aim of elucidating associations between circulating NG genotypes and antimicrobial resistance.
MethodsAll NG strains isolated from 2011 to 2015 in the Clinical Microbiology Service and Infection Control of the Basurto University Hospital of Bilbao (Spain) were included in this study. These NG strains were recovered from patients of the OSI Bilbao-Basurto, which covers a population of 356,000 (2016) inhabitants, and integrates the Basurto University Hospital, 25 Primary Care Centers and two specialized STI consultations. STI consultations are led by specialized staff (infectious disease doctors and clinical microbiologists) where a complete protocol for the diagnosis of STIs is carried out and a patient's clinical and sexual history are taken. To analyse the results of antimicrobial sensitivity and molecular typing, an isolate by location and patient was included. Regarding the analysis of the incidence of GI, the different episodes were registered. It was considered as a new episode when the same patient returned to the STI consultation with re-infection three months after a GI episode was treated and cured (no signs, nor symptoms nor presence of microorganisms).
Identification of NG strains was performed by means of Gram staining, cytochrome-oxidase test, catalase production and biochemical identification through the NH API system (BioMérieux, France) and confirmed by MALDI-TOF.
Clinical data such as patient gender, age, geographic origin, type of sexual relations maintained during the episode (heterosexual or homosexual) and anatomic site of infection, were recorded.
Antimicrobial susceptibility of NG isolates was carried out using the disc diffusion method in GC agar base (difco GC Medium Base, Becton Dickinson) with 1% Vitox (OXOID™), according to CLSI recommendations.10 Antimicrobials tested were penicillin, ciprofloxacin, tetracycline, azithromycin, gentamicin, spectinomycin, cefixime and ceftriaxone. Antimicrobial susceptibility to cefixime and ceftriaxone was also studied through agar dilution technique. Regarding to azithromycin, determination of MIC (minimum inhibitory concentration) was assessed by Etest. Results were interpreted according to the breakpoints established by EUCAST,11 except for gentamicin for which the breakpoints applied were those proposed by Bala et al.12 The results obtained were validated with NG strain ATCC 49226.10 β-Lactamase production was analysed by the Nitrocefin SRO112C (OXOID™) test.
Molecular typing was performed by NG multi-antigen sequence typing (NG-MAST) technique as described Martin et al.,13 which differentiates strains according to variations in internal fragments of two genes, the porB gene (producer of the PorB porin) and the tbpB gene (producer of the transferrin binding protein subunit B). Allele numbers and sequence types (STs) were assigned using the NG-MAST database (www.ng-mast.net). In addition, the alleles of the predominant STs (represented by ≥10 isolates) were classified in genogroups as described by Chisholm et al.14 Genogroups were named using the letter “G” followed by the predominant ST of each group.
Statistical analysis of the dataThe Kolmogorov–Smirnov test was used to verify the normality of the distribution when analysing the quantitative variables. To observe whether significant differences existed in the MIC50 throughout the years, Student's t test was used, or the Mann–Whitney U test if the normality of the distribution was not met. When analysing the relationship between categorical variables, Pearson's Chi-square test (χ2) was used or Fisher's exact test when any value of the calculation table was equal to zero. To explore associations between STs and Gs with the demographic characteristics of the patients and with the antimicrobial resistance, the “odds ratio” (OR) was calculated with a confidence interval (CI) of 95%. To verify if the ORs were significantly different, Pearson's χ2 test was used, except when any of the cells of the contingency table had a value equal to zero, in which case the Fisher exact test was conducted.
ResultsA total of 731 NG isolates were recovered from 576 patients. In 22 patients, two episodes of GI were detected and in 3 patients three episodes, resulting 604 episodes. There were 134 episodes in 2011 (38 cases per 100,000 inhabitants), 91 in 2012 (26/100,000), 84 in 2013 (24/100,000), 158 in 2014 (45.6/100,000) and 137 in 2015 (40.6/100,000). The majority of the patients (85.4%) were attended in the specialized STI consultations, due to appearance of clinical manifestations (68%), as part of a screening (14%) or due to risky sexual behaviours (18%). Only a small percentage came from Primary Care Centers (8.4%) or Emergency of Basurto University Hospital (6.2%), from where they were subsequently referred to STI consultations. 80.3% (463) of patients were male and 19.7% (113) female. The mean age of patients was 33 years (16–81 years). Furthermore, 35.4% were in the range of 25–34 years, followed by the range of 35–44 years (27.8%), 16–24 years (22.4%) and over 44 years (14.4%). Regarding patient nationality, 72.2% (416) were from Spain, 18.1% (104) from Latin America, 6.7% (39) from Africa, 1.8% (11) Europeans from countries other than Spain and 0.9% (6) from Asia. 43.9% (253) of patients were men who have sex with men (MSM) and among the females no homosexual relation was declared. Most of isolates were obtained from urethral samples (54.0%), followed by rectal (19.0%), pharyngeal (12.3%), endocervical (9.5%) and vaginal (5.2%).
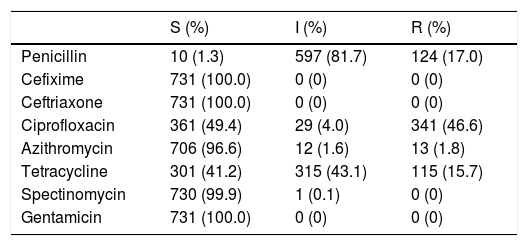
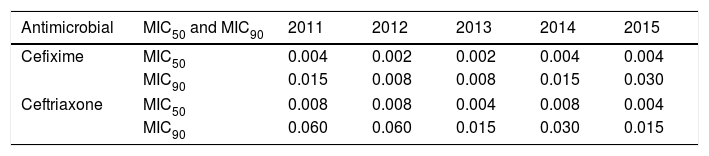
Ten isolates (1.3%) were susceptible to penicillin (Table 1) and 9.2% were resistant due to plasmid-mediated beta-lactamase production. All the strains were susceptible to cefixime and ceftriaxone. However, three strains presented MICs of 0.125mg/L to ceftriaxone, a dilution close to EUCAST breakpoint (R>0.125mg/L). Both cefixime and ceftriaxone presented a MIC50 of 0.004mg/L and the MIC90 was 0.030mg/L in cefixime and 0.015mg/L in ceftriaxone. Throughout the study a decrease of the MIC90 of ceftriaxone from 0.060mg/L to 0.015mg/L was observed (Table 2). The percentage of resistant strains to azithromycin was 1.3% and 3.4% in 2011 and 2012 respectively, and increased to 5.1% in 2013. However, it decreased significantly, being 0.5% in 2014 and 0.6% in 2015. Throughout the study, the percentage of resistant to azithromycin has been 1.8% and, in the year 2013, one strain showed a MIC of 96mg/L by Etest. Regarding ciprofloxacin 50.6% of the strains showed resistance or intermediate susceptibility. 41.2% of strains were susceptible to tetracycline, and 730 isolates (99.9%) were susceptible to spectinomycin. Regarding gentamicin, considering susceptible those strains with zone diameter ≥16mm, with intermediate susceptibility between 13–15mm and resistant ≤12mm, all strains were susceptible.12
Antimicrobial susceptibility of Neisseria gonorrhoeae strains studied.
| S (%) | I (%) | R (%) | |
|---|---|---|---|
| Penicillin | 10 (1.3) | 597 (81.7) | 124 (17.0) |
| Cefixime | 731 (100.0) | 0 (0) | 0 (0) |
| Ceftriaxone | 731 (100.0) | 0 (0) | 0 (0) |
| Ciprofloxacin | 361 (49.4) | 29 (4.0) | 341 (46.6) |
| Azithromycin | 706 (96.6) | 12 (1.6) | 13 (1.8) |
| Tetracycline | 301 (41.2) | 315 (43.1) | 115 (15.7) |
| Spectinomycin | 730 (99.9) | 1 (0.1) | 0 (0) |
| Gentamicin | 731 (100.0) | 0 (0) | 0 (0) |
S: susceptible; I: intermediate susceptibility; R: resistant.
Evolution of the MIC50 and MIC90 to cefixime and ceftriaxone throughout the period studied (2011–2015).
| Antimicrobial | MIC50 and MIC90 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|
| Cefixime | MIC50 | 0.004 | 0.002 | 0.002 | 0.004 | 0.004 |
| MIC90 | 0.015 | 0.008 | 0.008 | 0.015 | 0.030 | |
| Ceftriaxone | MIC50 | 0.008 | 0.008 | 0.004 | 0.008 | 0.004 |
| MIC90 | 0.060 | 0.060 | 0.015 | 0.030 | 0.015 | |
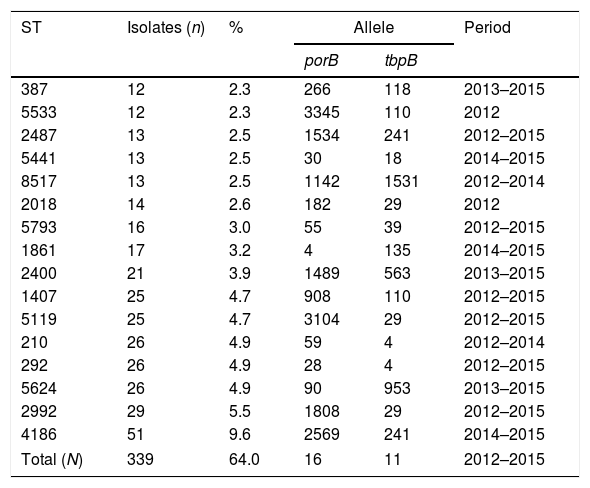
Molecular characterization was performed on 573 strains corresponding to the 2012–2015 period. In 42 strains had no database match (www.ng-mast.net), while the remainder matched to 110 different STs, with 83 porB alleles and 42 tbpB alleles. Fifty-three STs were represented by ≥2 isolates and 37 STs by ≥3 isolates. The most prevalent STs, represented by n≥10 isolates, are shown in Table 3. The STs 2992, 292, 1407 and 2487 were observed throughout the entire period studied (2012–2015). Conversely, ST5533 was just detected from January to April of 2012, affecting both heterosexual men, women and MSM and the ST4186, which was the most prevalent ST, was only observed in 2014 (n=30) and in 2015 (n=21).
Distribution of most prevalent STs (represented by n≥10 isolates).
| ST | Isolates (n) | % | Allele | Period | |
|---|---|---|---|---|---|
| porB | tbpB | ||||
| 387 | 12 | 2.3 | 266 | 118 | 2013–2015 |
| 5533 | 12 | 2.3 | 3345 | 110 | 2012 |
| 2487 | 13 | 2.5 | 1534 | 241 | 2012–2015 |
| 5441 | 13 | 2.5 | 30 | 18 | 2014–2015 |
| 8517 | 13 | 2.5 | 1142 | 1531 | 2012–2014 |
| 2018 | 14 | 2.6 | 182 | 29 | 2012 |
| 5793 | 16 | 3.0 | 55 | 39 | 2012–2015 |
| 1861 | 17 | 3.2 | 4 | 135 | 2014–2015 |
| 2400 | 21 | 3.9 | 1489 | 563 | 2013–2015 |
| 1407 | 25 | 4.7 | 908 | 110 | 2012–2015 |
| 5119 | 25 | 4.7 | 3104 | 29 | 2012–2015 |
| 210 | 26 | 4.9 | 59 | 4 | 2012–2014 |
| 292 | 26 | 4.9 | 28 | 4 | 2012–2015 |
| 5624 | 26 | 4.9 | 90 | 953 | 2013–2015 |
| 2992 | 29 | 5.5 | 1808 | 29 | 2012–2015 |
| 4186 | 51 | 9.6 | 2569 | 241 | 2014–2015 |
| Total (N) | 339 | 64.0 | 16 | 11 | 2012–2015 |
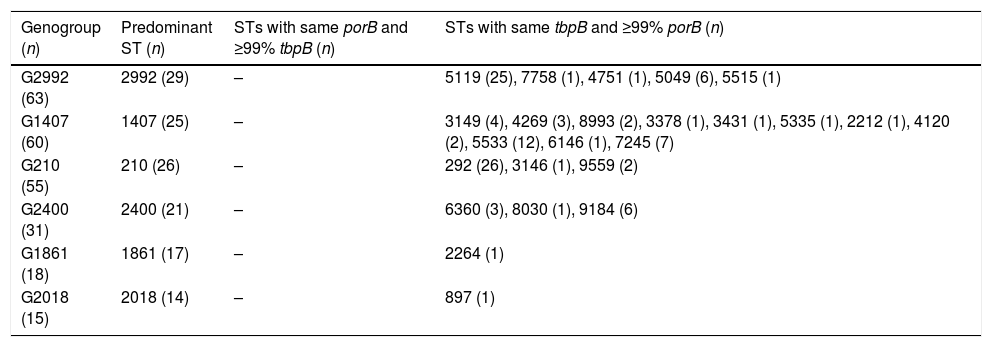
Six genogroups were defined encompassing 242 (42.23%) of all isolates (Table 4). G2992 was the largest and G1407 grouped the higher number of different STs.
Genogroups obtained by grouping the STs based on the similarity of the porB and tbpB alleles and the STs that form them.
| Genogroup (n) | Predominant ST (n) | STs with same porB and ≥99% tbpB (n) | STs with same tbpB and ≥99% porB (n) |
|---|---|---|---|
| G2992 (63) | 2992 (29) | – | 5119 (25), 7758 (1), 4751 (1), 5049 (6), 5515 (1) |
| G1407 (60) | 1407 (25) | – | 3149 (4), 4269 (3), 8993 (2), 3378 (1), 3431 (1), 5335 (1), 2212 (1), 4120 (2), 5533 (12), 6146 (1), 7245 (7) |
| G210 (55) | 210 (26) | – | 292 (26), 3146 (1), 9559 (2) |
| G2400 (31) | 2400 (21) | – | 6360 (3), 8030 (1), 9184 (6) |
| G1861 (18) | 1861 (17) | – | 2264 (1) |
| G2018 (15) | 2018 (14) | – | 897 (1) |
ST: sequence type; G: genogroup.
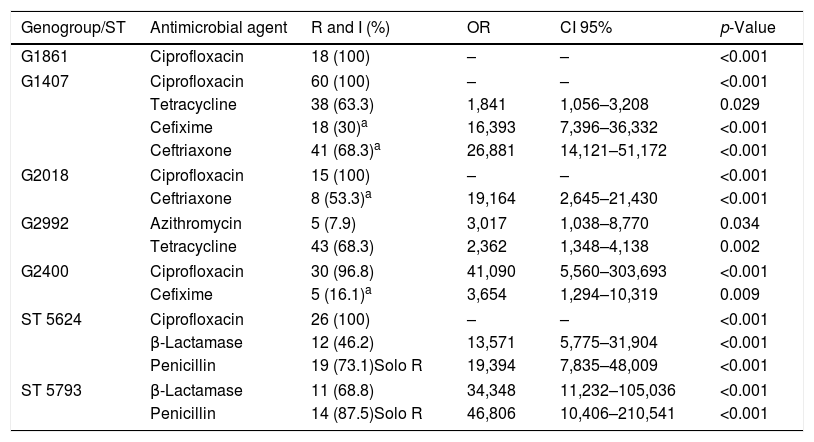
Based on gender category, G1861 (OR: 4.763; p=0.001), G1407 (OR: 2.377; p=0.009), G2018 (OR: 4.667; p=0.004) and ST387 (OR: 7.038; p=0.001) were more prevalent in females, while ST5624 was only present in males (p=0.016). With regard to age category, G1861 was found more frequently in patients aged under 24 (OR: 4.281; p=0.002), whereas G2018 (OR: 4.860; p=0.004) and G2992 (OR: 2.671; p=0.005), were more associated with patients aged over 44. Regarding sexual orientation, G1407 was more common among heterosexual patients (OR: 4.574; p=0.004) and all isolates of G2018 were detected in heterosexual patients (p=0.002). On the other hand, G2992 (OR: 4.734; p=0.004) and G2400 (OR: 16.488; p<0.001) were more frequently observed in MSM. An association was not found between patient geographic location and the genogroups or STs described. It was also observed that the strains belonging to G1407 presented high MICs (MIC≥0.030mg/L) to cefixime (OR: 16.393) and ceftriaxone (OR: 26.881). G2018 was associated with strains with high MICs to ceftriaxone (OR: 19.164) and azithromycin resistance (OR: 3.017) was associated with G2992. G2400 showed a high ratio of isolates with resistance to ciprofloxacin (OR: 41.090) and a moderate ratio with a high MICs to cefixime (OR: 3.654). All the isolates of G1861, G2018, G1407 and ST5624 showed resistance or intermediate susceptibility to ciprofloxacin (Table 5), based on a significant association detected by means of Fisher's exact test (p<0.001).
Relationship between the genogroups and the principal STs with antimicrobial resistance.
| Genogroup/ST | Antimicrobial agent | R and I (%) | OR | CI 95% | p-Value |
|---|---|---|---|---|---|
| G1861 | Ciprofloxacin | 18 (100) | – | – | <0.001 |
| G1407 | Ciprofloxacin | 60 (100) | – | – | <0.001 |
| Tetracycline | 38 (63.3) | 1,841 | 1,056–3,208 | 0.029 | |
| Cefixime | 18 (30)a | 16,393 | 7,396–36,332 | <0.001 | |
| Ceftriaxone | 41 (68.3)a | 26,881 | 14,121–51,172 | <0.001 | |
| G2018 | Ciprofloxacin | 15 (100) | – | – | <0.001 |
| Ceftriaxone | 8 (53.3)a | 19,164 | 2,645–21,430 | <0.001 | |
| G2992 | Azithromycin | 5 (7.9) | 3,017 | 1,038–8,770 | 0.034 |
| Tetracycline | 43 (68.3) | 2,362 | 1,348–4,138 | 0.002 | |
| G2400 | Ciprofloxacin | 30 (96.8) | 41,090 | 5,560–303,693 | <0.001 |
| Cefixime | 5 (16.1)a | 3,654 | 1,294–10,319 | 0.009 | |
| ST 5624 | Ciprofloxacin | 26 (100) | – | – | <0.001 |
| β-Lactamase | 12 (46.2) | 13,571 | 5,775–31,904 | <0.001 | |
| Penicillin | 19 (73.1)Solo R | 19,394 | 7,835–48,009 | <0.001 | |
| ST 5793 | β-Lactamase | 11 (68.8) | 34,348 | 11,232–105,036 | <0.001 |
| Penicillin | 14 (87.5)Solo R | 46,806 | 10,406–210,541 | <0.001 | |
R: resistant; I: intermediate susceptibility; OR: odds ratio; CI 95%: confidence interval of 95%.
This study is one of the few published in Spain, along with the ones published in Barcelona15,16 and Almería,17 that analyses associations between STs of NG and antimicrobial resistance profile.
The incidence of GI observed, with a minimum of 24 cases per 100,000 inhabitants (2013) and a maximum of 45.6 cases per 100,000 inhabitants (2014), is much higher than that observed in Spain during the same period of time by the National Epidemiological Monitoring Network (RENAVE), who recorded a minimum of 5.7 cases per 100,000 inhabitants (2011) and a maximum of 11.4 cases per 100,000 (2015)18 or by ECDC who detected a rate of 18.8 cases per 100,000 inhabitants of the EU/EEA (2016).1 These data indicate that GI could be underestimated due to a suboptimal diagnosis, a lack of screening in asymptomatic patients and/or incomplete diagnostics in cases of STD.
In the present study, male patients are predominantly involved, accounting for 80.3% of the episodes. A similar percentage was observed in the RENAVE network in 2015 (88%)18 and by the EU/EEA in 2016 (70%).1 Most of the cases were produced in patients aged 25–44 while patients under 25 years comprised 22.4% of cases, lower than the 36% observed by the ECDC.1 The MSM group accounted for 43.9% of the patients with GI, which is in close agreement with the rate observed by the ECDC in 2016 (46%).1
The high rates of resistance to penicillin, ciprofloxacin and tetracycline are similar to those observed in other studies carried out in Spain15,17 and the ECDC.19 This fact reinforces the instructions of the ECDC and WHO to not use these antimicrobials for the empirical treatment of the GIs.4,19 NG strains resistant to cefixime and ceftriaxone have been reported from several countries, including Spain.20 However, in the present study there was no observation of any strain resistant to these antibiotics, for which reason the dual therapy based on ESC plus azithromycin recommended by ECDC and WHO continues to be the best therapeutic option to treat GI in our health area. Since antimicrobial resistance is the principal challenge associated with this microorganism, institutions should determine a unique breakpoint to define resistance. As a matter of fact, three isolates showed MIC to ceftriaxone of 0.125mg/L, a dilution below the resistance threshold according to the breakpoints published by EUCAST (R>0.125mg/L), but far from being considered resistant according to CLSI (R>0.25mg/L). Azithromycin constitutes a front-line option in dual therapy along with ceftriaxone with the objective of eradicating the possible coexisting infection by C. trachomatis and due to its involvement in the treatment of pharyngeal gonorrhoea. However, probably due to the generalised use that has been made of this antibiotic to treat this type of infections as well as for respiratory infections, resistance to azithromycin has been developed, as it was shown in ECDC published percentages (7.5% in 201719). In our study, in contrast, a clear decline of strains with intermediate susceptibility or resistance to azithromycin from 8.3% in 2013 to 0.7% in 2015 was detected. From all isolates tested only one was resistant to spectinomycin and no resistance was observed to gentamicin according to the criteria used. These results could suggest the use of these antibiotics as an alternative treatment of GI. However, the national therapeutic guidelines do not recommend them because spectinomycin is not available in Spain and its effectiveness is lower in pharyngeal and rectal infections,3 and the WHO does not consider the use of gentamicin due to lack of data supporting its in vivo efficacy in GI.4
ST1407 and ST2992 stand out for being prevalent in Spain15–17 and in the Euro-GASP study (2010),14 in which they observed that ST1407 was the predominant ST in 13 countries of the EU/EEA. ST387, one of the most frequent in our study was also detected by the Euro-GASP14 and in the study conducted in Almería.17 Regarding the ST4186, the most prevalent in our study, in the study of Chisholm et al.14 it is not among the most prevalent ST in Europe and has not been published in Spain, which indicates that it could be an ST endemic to our area. In addition, ST4186 does not belong to any genogroup, which reveals that it is a new clone that does not descend from the most prevalent. Given that ST5533 was isolated in a determined period of time (January to April of 2012) and was not reported in other studies as a frequent ST, it could be an outbreak of GI in our area. In this study 42 strains were not detected in NG-MAST database suggesting that they could be newly emerging or previously not characterized STs.
Regarding the genogroups, G2992 (n=63) and G1407 (n=60) were the largest. These were also the largest in the Euro-GASP study in 201014 and they were detected in other European studies.16,21–24 G2400 (n=31) was detected in Italy,21 France22 and other areas of our country.16 The association between genogroups G2992 and G2400 and the MSM collective also were observed by Euro-GASP group.14 In addition, G2992 was widely detected throughout the study period, which could indicate the presence of an outbreak of GI within MSM. Another important fact that the analysis of the genogroups and the antimicrobial resistance provides us is the significant relationship found between G2992, and the presence of resistance to azithromycin, an association already published by a Dutch study (2008–2015),25 which indicates that the presence of G2992 could become a public health problem in our area. The relationship between infection by G1407 and the presence of high MICs (MIC≥0.030mg/L) to cefixime and ceftriaxone has already been described in several European studies.14,16,23–26 These results show how valuable molecular typing is in predicting the NG antimicrobial resistance, since the use of cefixime as the empirical treatment of the GI produced by G1407 seems inappropriate.14 The relationship between G2400 and high MICs for cefixime has not been published to date in any European study. Nonetheless, in USA in 2014 a study demonstrated a significant relationship between this genogroup and the presence of strains with intermediate MICs (0.032–0.0394mg/L) to cefixime and ceftriaxone27 and in Canada G2400 was correlated with reduced susceptibility to cefixime and ceftriaxone.28 We found a significant relationship between G2400 and resistance to ciprofloxacin, a fact already published in studies conducted in Greenland29 and in Canada.28 In this work we describe for the first time the presence of G1861 and G2018 as well as their relationship with resistance to ciprofloxacin and association of G2018 with the presence of high MICs to ceftriaxone.
In conclusion, by analysing the associations between the different STs and Gs detected and antimicrobial resistance, we obtained very valuable information. G1407, G2400 and G2018, which encompassed 14.5% of the strains isolated, are significantly related to high MICs of cefixime and/or ceftriaxone. Furthermore, the resistance to azithromycin shown by the strains belonging to G2992, indicates that a high number of resistant strains or with high MICs to the antimicrobials recommended are circulating in our environment, which could cause treatment failures with cefixime and ceftriaxone in the future and azithromycin at present. In this context, it seems necessary to develop novel antimicrobials or to design new antimicrobial therapies based on drugs that show their efficacy against GI, such as gentamicin. We must highlight the importance of STI surveillance especially in the homosexual population, since two of the most prevalent genotypes in our area, G2992 and G2400, are significantly associated with MSM.
Funding sourceThe present investigation has received a scholarship from OSI Bilbao- Basurto.
Conflict of interestsNone of the authors show a conflict of interest.
We would like to acknowledge to the Department of Microbiology of Basque Country University (Universidad del País Vasco; UPV/EHU) and the Research Department of the University Hospital of Basurto.