Cutaneous leishmaniasis (CL) is an infection with a global distribution, considered a "neglected" disease by the World Health Organisation, and which affects up to one million people each year. Its management represents a therapeutic challenge due to the toxicity of the drugs used and the possible development of resistance to them. In this context, American infectious disease and tropical medicine guidelines1 do not recommend any particular first-line treatment and advocate for a personalised choice depending on the characteristics of the lesion, the parasite and the host. We report the case of a patient with CL of the face treated with imiquimod 3.75%, a concentration not previously reported in the treatment of CL.
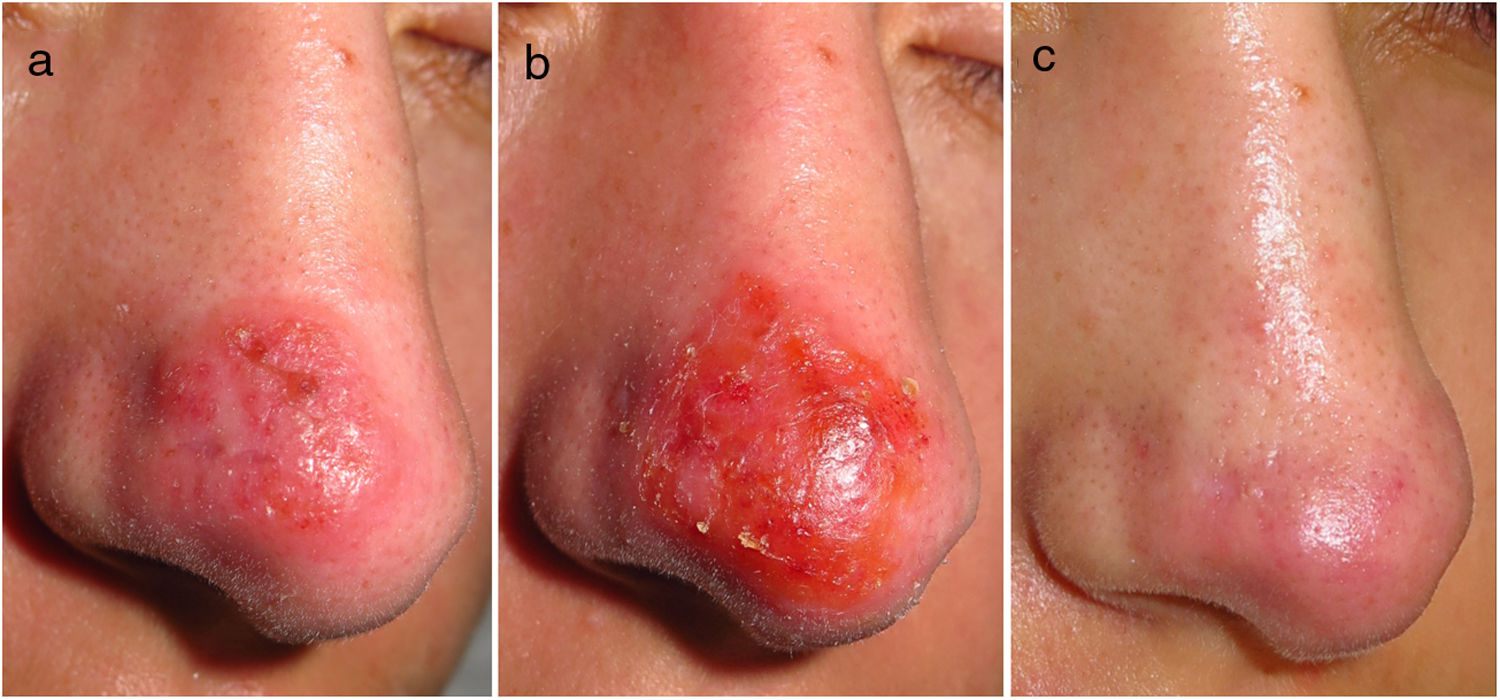
A 21-year-old Spanish female, with no disease history of note or recent travel, sought treatment for an asymptomatic, firm, erythematous–oedematous plaque on the tip of her nose, measuring 2 cm in diameter, which had developed two months earlier (Fig. 1a). Histology testing showed lymphohistiocytic dermal inflammatory infiltrate. Molecular testing (polymerase chain reaction) confirmed Leishmania infantum infection. Faced with the difficulty in infiltrating intralesional meglumine antimoniate, given the lesion's induration and location, a decision was made to start imiquimod 3.75% (Zyclara, Meda Pharma) on alternate days. The patient had an inflammatory reaction (Fig. 1b) that was well-tolerated, and so the treatment was continued for two months. After four months of follow-up, no relapse has been seen and the lingering aesthetic defect is minimal (Fig. 1c).
Course of cutaneous leishmaniasis: before, during and after imiquimod 3.75%. (a) Erythematous–oedematous plaque on the patient's nose prior to starting treatment. (b) Local inflammatory reaction three weeks after starting imiquimod. (c) The patient's nose with no leishmaniasis lesions four months after ending treatment.
Imiquimod is a topical immunomodulator approved for the treatment of viral warts and premalignant cutaneous lesions. In CL, imiquimod stimulates secretion of interferon gamma by CD4 T helper-1 lymphocytes by activating macrophages to destroy amastigotes.2 This mechanism of action would reduce the development of resistance, and we believe that it could be useful for treating areas of subclinical infection (i.e. apparently healthy areas surrounding the lesion that are actually infected), as in its use for the treatment of premalignant lesions. Indeed, in our patient, the inflammatory reaction in the area treated (Fig. 1b) was slightly greater than in the clinically affected area (Fig. 1a). Imiquimod 3.75% causes a more controlled inflammatory reaction than imiquimod at higher concentrations. It was formulated to improve problems of tolerability and adherence to the drug at a concentration of 5%. This reduced inflammatory reaction also leads to a better aesthetic outcome, making it particularly interesting as a treatment for facial lesions. There is no evidence on the use of imiquimod at low concentrations to treat CL.
Seeberger et al.3 found that imiquimod 5% showed temporary effectiveness as a treatment for CL. Crawford et al.4 concluded that imiquimod 5% was superior to intralesional pentavalent antimoniate, both in monotherapy and in combination with the latter. Other authors5–7 have demonstrated its effectiveness at concentrations of 5% and 7.5%, as co-adjuvant treatment to pentavalent antimoniates. However, two other similar studies8,9 did not succeed in yielding the same results. In addition, isolated cases of response to imiquimod in CL resistant to first-line treatments have been published, suggesting that it could be an alternative should the parasite develop resistance.10
Facial CL should always be treated with a view to minimising the aesthetic consequences.1 In cases of localised CL in immunocompetent patients in the Old World, local options are the treatments of choice.1 In our case, imiquimod 3.75% was chosen to limit the inflammatory reaction in an aesthetically significant area. Our patient showed highly satisfactory results and good drug tolerance.
Given the variety of scenarios possible in CL and the disadvantages associated with the treatments available, the outcome in this case suggests that imiquimod at low concentrations may be a good treatment option in cases of CL with no criteria for systemic treatment, in aesthetically compromised areas, especially where the infiltration of pentavalent antimoniates is problematic or there is resistance to them.
Please cite this article as: Marti-Marti I, Alsina M, Giavedoni P, Fuertes I. Leishmaniasis cutánea facial tratada con imiquimod al 3,75%. Enferm Infecc Microbiol Clin. 2021;39:108–109.