Co-infection with hepatitis C (HCV) and B (HBV) viruses is common in clinical practice. We report a case of fulminant hepatic failure as a consequence of reactivation of HBV in a patient with human immunodeficiency virus (HIV) after HCV treatment.
This was a 53-year-old man diagnosed in 1998 with HIV infection following detection of pulmonary tuberculosis, with a good current response to antiretroviral therapy. In addition, he had chronic HCV genotype 1a infection and a history of previous HBV infection with positive IgG (anti-HBc IgG) core antibody, negative surface antigen (HBsAg) and antibodies (anti-HBs) and undetectable levels of HBV DNA, prior to initiation of treatment with direct-acting antivirals (DAA).
For his HIV infection, the patient had previously followed several treatment regimens with multiple failures and development of resistance. In 2011, he was prescribed treatment with tenofovir (TDF), abacavir and ritonavir-boosted atazanavir. The TDF was subsequently withdrawn after a slight worsening of his renal function, and the treatment was simplified to darunavir/cobicistat monotherapy. Since then, his viral load has been undetectable and his CD4 T-cell lymphocytes 500–800/mm3 (CD4 T lymphocytes 800/mm3 at most recent testing, prior to starting HCV treatment).
In October 2013, the patient started treatment for HCV according to the regimen pegylated interferon alpha-2a 180μg, one weekly injection, and ribavirin 1000mg daily (3-0-2), resulting in a 3-log decrease in HCV viral load by week 4 of treatment. The idea of adding first-generation direct-acting antivirals (telaprevir) was proposed, but the patient refused to continue treatment because of poor tolerance to interferon.
In December 2015, his HCV viral load was 2,181,330.11IU/ml with a liver stiffness measurement of 12kPa. Since we already had DAA, he was started on treatment with sofosbuvir and ledipasvir for 12 weeks.1 In week 2, his viral load had dropped to 284.51IU/ml, by week 4 it was down to 30.45IU/ml and by week 8 it was less than 15IU/ml, with a sustained viral response at week 4 post-treatment.
A month later, the patient consulted with abdominal pain, nausea and jaundice, with total bilirubin 10.98mg/dl, direct bilirubin 8.75mg/dl, GOT 1025.40IU/l, GPT 642IU/l and INR 1.30. An HBV viral load of 6,193,455.96IU/ml was found, with positive HBsAg, anti-HBc IgM and HBeAg serology. His HCV viral load remained undetectable, and hepatitis D virus was negative. We were therefore dealing with acute hepatitis caused by HBV.
The patient was started on treatment with entecavir (TDF had already been discontinued due to impaired renal function); however, he made poor progress and was included in a pre-liver transplant study. Despite the treatment, the patient became worse and was transferred to the Intensive Care Unit, where he continued to deteriorate and died as a result of acute liver failure.
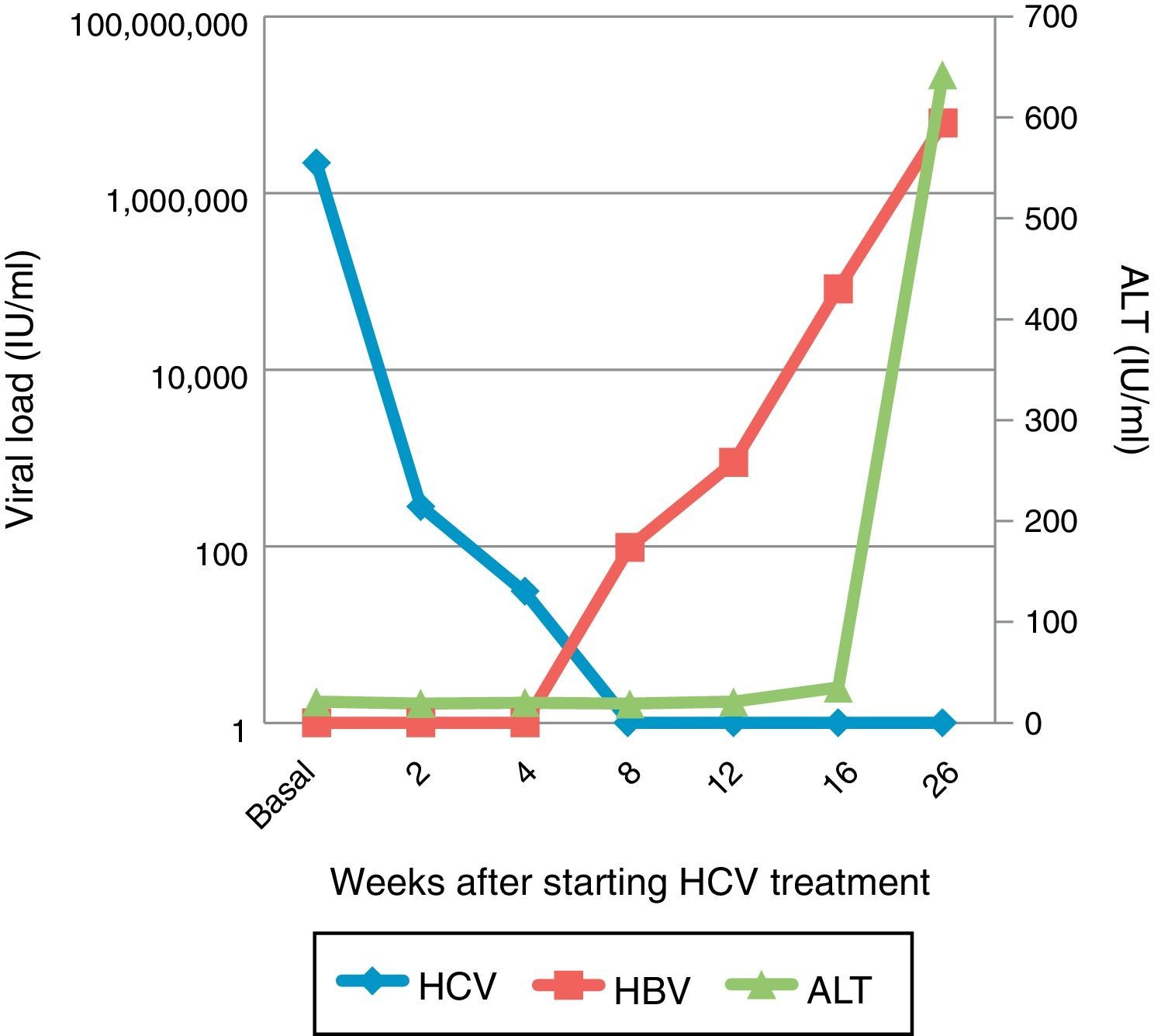
The patient's HBV viral load was requested from samples stored during the HCV treatment; it was undetectable at the start of treatment and at week 2, but progressively increased to 98.80IU/ml after 8 weeks of treatment and then to 82,700IU/ml at week 4 post-treatment (Fig. 1).
The risk of reactivation of HBV in the context of immunosuppressive therapy is well known.2 However, several cases of HBV reactivation following DAA treatment in HCV-HBV co-infected patients have been reported, and although most have had no clinical repercussions and a good response to nucleotide/nucleoside analogue treatment,3,4 there was one 59-year-old patient with genotype 1b who developed acute fulminant HBV hepatitis in week 11 of treatment and had to have a liver transplant.5
In HCV-HBV co-infected patients, HBV DNA is usually low or undetectable,6 although it can fluctuate, with HCV being the main cause of chronic liver activity. Different theories have been put forward to explain the inhibition of HBV replication in co-infected patients.7 The first is that there seems to be a direct interaction between the two viruses, with HCV inhibiting HBV replication, but the inhibition stops when the HCV is treated. Another theory is the possible increase in the space available for replication of the B virus after HCV treatment. The most accepted theory for the moment, however, is that chronic HCV replication produces an immune status in the body which helps to control HBV replication, but that this is interrupted by treatment with DAA.
In conclusion, this is the first reported case of fulminant HBV-related liver failure in the context of treatment with the new DAAs in a patient with HIV. The patient had been pre-treated with IFN for 4 weeks with no evidence of reactivation of HBV, so it might be argued that in view of their action against HBV, IFN-based therapies might be safer in this regard, at the expense of more adverse effects. In addition, there was no evidence of reactivation when TDF was withdrawn, which gives more weight to a relationship between the DAA treatment and the reactivation of HBV. We do not know whether the HBV reactivation or poor clinical outcome might be related to HIV infection, particularly when patients are not on 3TC/TDF treatment. Moreover, it must be taken into account that a large number of co-infected patients are treated with nucleoside/nucleotide analogues, which could prevent the reactivation of HBV. Also, our patient had no anti-HBs, so it would be interesting to assess the isolated risk in people with negative anti-HBs. At present, we are still uncertain about how to optimally manage these situations.7–9 We therefore believe that protocols are required for strict monitoring of patients taking DAAs for HCV who have old or active HBV infection (isolated HBcAb or HBsAg), regardless of the stage, genotype or type of DAA, in order to prevent the risk of HBV reactivation in these situations.
Please cite this article as: Paniagua-García M, López-Hernández I, Fernández-Cuenca F, Ríos-Villegas MJ. Hepatitis aguda fulminante por virus B durante el tratamiento del virus de la hepatitis C con antivirales de acción directa en paciente infectado con VIH. Enferm Infecc Microbiol Clin. 2017;35:681–682.