To study the spatio-temporal distribution of cases of invasive pneumococcal disease (IPD) due to serotypes resistant to erythromycin and its relationship with community consumption of macrolides and childhood vaccination coverage.
MethodsWe selected IPD cases in adults over 59 years old, residents in the Community of Madrid (MC), notified in the period 2007–2016. The variables studied were obtained from the Vaccination Information Systems and the Pharmaceutical Service. The cut-off point (minimum inhibitory erythromycin concentration > 0.5 mg/L) of the EUCAST classification was used to define erythromycin resistant serotypes. We used JointPoint to estimate the incidence trends by erythromycin resistant serotypes included in the 13-valent vaccine (STPCV13) and not included in it (STnoPCV13). The association of these incidences with the community consumption of macrolides and vaccination coverage was made using Poisson models. Statistical scanning was used for the detection of temporal-spaces clusters of cases.
Results1936 cases were identified, of which 427 erythromycin resistant serotypes were identified. The incidence of all cases due to resistant serotypes was decreasing (AAPC: −5,40%). During the period studied, the incidence of cases due to erythromycin resistant STPCV13 was decreasing with an annual percentage change (APC): −13.8 and was inversely associated with childhood vaccination coverage (IRR 0.641), while that of cases due to erythromycin resistant STnoPCV13 was ascending (APC): 4.5; and was not associated with coverage. 1 cluster was detected by STnoPCV13 and none by STPCV13 after the date of inclusion of the 13-valent in the childhood vaccination calendar.
ConclusionsThe decrease in IPD due to resistant STPCV13 was associated with an increase in childhood vaccination coverage. The presence of clusters due to STnoPCV13 after the date of inclusion of the 13-valent vaccine in the childhood vaccination calendar indicates serotypes replacement. The increase in cases of resistant STnoPCV13 could be related to the replacement of vaccine serotypes in nasopharyngeal colonization, facilitated by the consumption of macrolides still at high levels in MC.
Estudiar la distribución espacio-temporal de los casos de enfermedad neumocócica invasora (ENI) por serotipos resistentes a eritromicina y su relación con el consumo comunitario de macrólidos y la cobertura vacunal infantil.
MétodosSe seleccionaron los casos de ENI en mayores de 59 años residentes en la Comunidad de Madrid (CM) notificados en el período 2007–2016. Las variables estudiadas fueron obtenidas de los sistemas de información vacunal y de Prestación Farmacéutica. Se utilizó el punto de corte (concentración mínima inhibitoria de eritromicina > 0,5 mg/L) de la clasificación de EUCAST para definir los serotipos resistentes a eritromicina. Mediante JointPoint se estimaron las tendencias de las incidencias de casos por serotipos resistentes a eritromicina incluidos en la vacuna trecevalente (STVCN13) y no incluidos (STnoVCN13). La asociación de esas incidencias con el consumo comunitario de macrólidos y la cobertura vacunal se hizo mediante modelos de Poisson. Para la detección de clústeres espacio-temporales se utilizó el estadístico Satscan.
ResultadosSe identificaron 1936 casos, de los que en 427 se identificaron serotipos resistentes a eritromicina. La incidencia de todos los casos por serotipos resistentes fue descendente (AAPC: −5,40%). La incidencia de casos por STVCN13 resistentes a eritromicina fue descendente con un porcentaje anual del cambio (APC: −13.8) y estuvo asociada inversamente a la cobertura vacunal infantil (IRR 0.641), mientras que la de casos por STnoVCN13 resistentes a eritromicina fue ascendente (APC: 4.5) y no se asoció con la cobertura. Se detectó 1 clúster por STnoVCN13 y ninguno por STVCN13 tras la inclusión de la trecevalente en el calendario vacunal infantil.
ConclusionesEl descenso de ENI por STVCN13 resistentes se asoció con el incremento de la cobertura vacunal infantil. La presencia de clústeres de casos por STnoVCN13, en el periodo posterior a la inclusión de la VCN13, indica reemplazo de serotipos. El aumento de los casos por STnoVCN13 resistentes podría estar relacionado por el reemplazo de los serotipos vacunales en la colonización nasofaríngea, facilitada por el consumo de macrólidos todavía a niveles altos en la CM.
Antibiotic resistance is a significant public health problem, with an as yet undefined clinical impact1. Streptococcus pneumoniae is the most common cause of respiratory infections. There are more than 100 serotypes, of which approximately 20 cause more than 90% of cases of invasive pneumococcal disease (IPD)2. The first resistant serotypes appeared in the areas with the highest antibiotic consumption in South Africa in the late 1970s3. Initial resistance to β-lactam antibiotics soon spread to other antibiotics, such as the macrolides, regarded as the first-line treatment in upper respiratory infections.
In Europe, the first resistant pneumococci appeared in the 1980s. The incidence of cases due to macrolide-resistant serotypes has remained stable in recent years. In Spain, the proportion of cases (21.6% in 2017) is lower than other European countries, such as France (23.35%), Romania (27.6%), Bulgaria (27.6%) or Italy (23.4%)4.
The most important population risk factor for antibiotic resistance is community antimicrobial consumption. Studies indicate that areas with the highest antibiotic consumption have an increased incidence of resistant cases5. Another influencing factor is systematic childhood vaccination with the pneumococcal conjugate vaccine (PCV)6,7. The PCV acts by decreasing pharyngeal colonisation by Streptococcus pneumoniae in children under two years of age, who are considered to be the main reservoir and transmitters to the rest of the population8.
In 2006, the heptavalent vaccine (PCV7), which covers serotypes 4, 6 B, 9V, 14, 18C, 19F and 23F, was included as part of the Community of Madrid (CM)’s childhood vaccination schedule. As a consequence of the serotype replacement observed, in 2010 it was replaced by the 13-valent vaccine (PCV13), with six additional serotypes (1, 5, 7F, 3, 6A and 19A)9.
Although the incidence of IPD has diminished in the last 10 years10, a similar geographic distribution between cases would be expected due to erythromycin-resistant serotypes and macrolide consumption11, as well as a different spatiotemporal distribution between cases due to serotypes resistant and sensitive to erythromycin.
The objective of this study is to analyse the spatiotemporal distribution of IPD cases due to erythromycin-resistant serotypes in the CM during the period between 2007 and 2016 and the relationship with the community consumption of macrolides and childhood vaccination coverage.
MethodsStudy scope and periodIPD cases in residents of the CM over 59 years of age reported during the 2007–2016 period. This period was subdivided into the following periods: pre-PCV13 (2007–2009) and PCV13 (2011–2016). PCV13 was included as part of the CM's childhood vaccination schedule in 2010.
Case definitionThe IPD case definition of the Epidemiological Surveillance Network (Red de Vigilancia Epidemiológica) was used, which requires that one of the following laboratory criteria be met: isolation, detection S. pneumoniae of DNA or antigen in samples from normally sterile sites. The criterion that was fulfilled to define a case of IPD in the present study was the isolation of S. pneumoniae in a sterile sample. Erythromycin resistance was defined as an increase in the minimum inhibitory concentration of erythromycin greater than 0.5 mg/l in the antibiogram.
Data sources and variablesCases reported to the Notifiable Disease Surveillance System (Sistema de Vigilancia de Enfermedades de Declaración Obligatoria [EDO]) were included. This system collects demographic, clinical, epidemiological and microbiological variables using a standardised form. The Regional Public Health Laboratory (Laboratorio Regional de Salud Pública) identified serotypes using the Quellung reaction and the antibiotic sensitivity analysis according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) classification12. The cases were classified into four groups according to the type of serotype: (1) any serotype (total ST), (2) serotypes included in the PCV13 (STPCV13), (3) serotypes additional to the PCV7, included in the PCV13 (STPCV13not7) and (4) serotypes not included in the PCV13 (STnotPCV13).
The Vaccine Information System (Sistema de Información Vacunal) provided the number of vaccinated cases per year. Annual vaccination coverage (VC13) was calculated as the proportion of primary vaccinated children in the two-year-old cohort and was categorised into three levels (<85%, 86%–90%, and >90%).
Furthermore, the Pharmaceutical Provision Information and Analysis System (Sistema de Información y Análisis de la Prestación Farmacéutica) provided the community antibiotic consumption, expressed as the defined daily dose (DDD) pertaining to the population of Madrid over 59 years of age. The DDD groups consumption per prescribed active ingredient (ATC) of a family of antibiotics (macrolides)13. Subsequently, the DDD per 1,000 inhabitants per day (DDD/1,000 inhabitants/day) of over-59s was calculated13. The following formula was used: DDD/1,000 inhabitants/day = (DDD*1,000)/population*365. The reference population was obtained from the Continuous Register of the Statistics Institute (Padrón Continuo del Instituto de Estadística) of the CM.
Data analysisThe trend analysis was performed using Joinpoint Trend Analysis models, which calculated the Annual Percent Change (APC) between the cumulative incidence from one year to the next. The Average Annual Percent Change (AAPC) of the accumulated incidences throughout the study period was also estimated. Both estimates were made for the four groups of cases defined by serotype.
The mean annual incidences of the pre-PCV13 and PCV13 periods were compared for each group of cases using the Incidence Rate Ratio (IRR) obtained from the Poisson models.
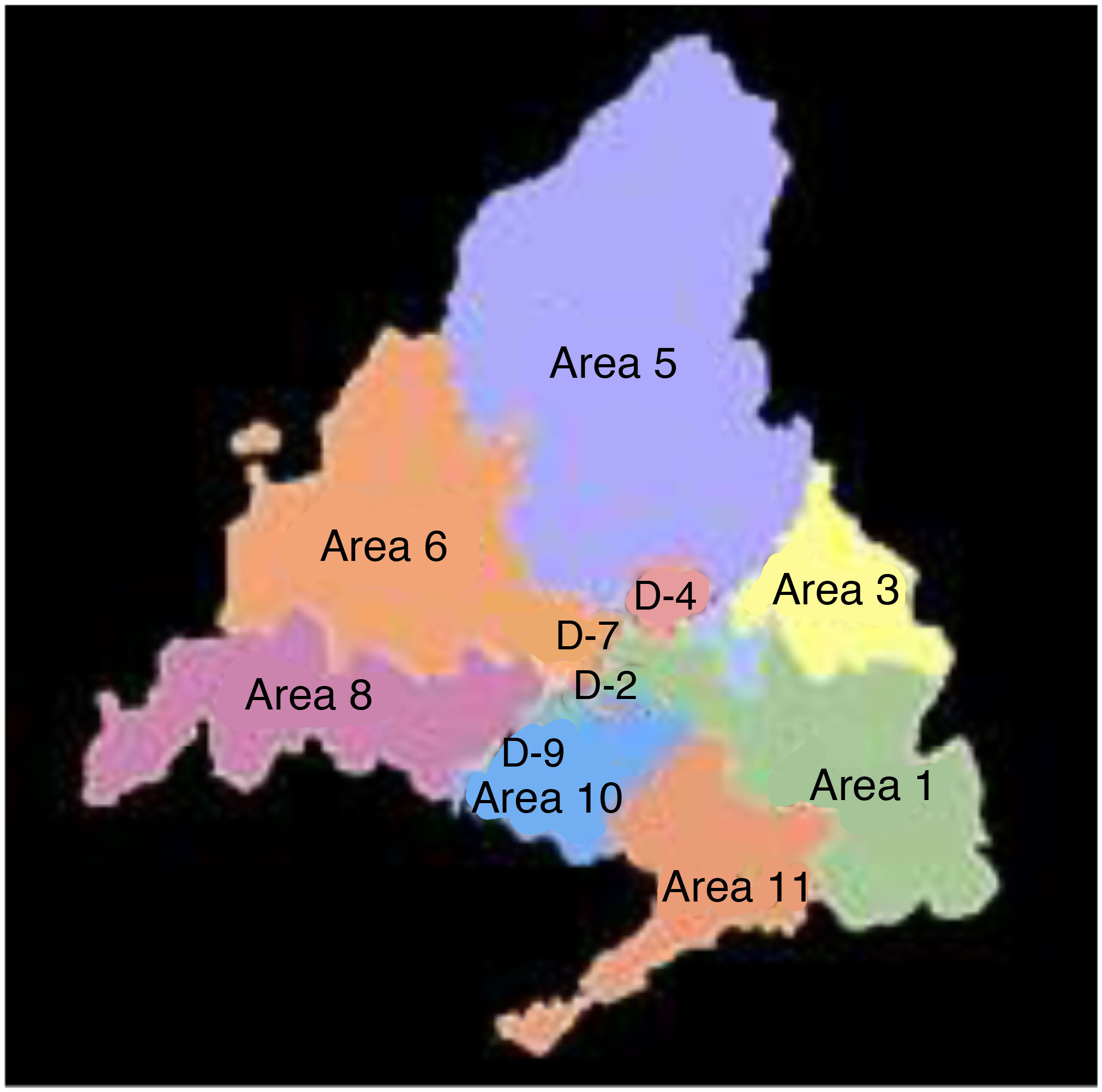
To carry out the spatial analysis, the cumulative incidence of cases was calculated for each one of the 11 historical health areas (Fig. 1) in the pre-PCV13 and PCV13 periods for the four groups of defined cases. Incidences were smoothed using the “empirical Bayes” technique and represented on maps. This technique calculates the weighted average between the gross cumulative incidence of each area and the overall mean cumulative incidence proportional to the size of the population. In this way, areas with small populations have values closer to the mean and those with large populations values closer to their raw incidence. Macrolide intake (DDD/1,000 inhabitants/day) was also calculated by health area for both periods.
The detection of clusters of cases was carried out using the SaTScan™ statistics software, developed by Kulldorff, following a Poisson distribution14. This method consists of creating a cylindrical window that continuously changes centre, radius and height, scanning the areas of Madrid. We restricted the radius of the window to the average distance between the centroids of the areas. For each centroid of an area, the radius varied from zero to 25 km. The height of the cylinder represented the time dimension, between 1 and 10 years. The most probable cluster was the one with more cases than expected in the established spatiotemporal dimensions. The p value was obtained through Monte Carlo simulations (999 repetitions) with a 95% confidence interval.
The association of annual cumulative incidence of IPD with VC13 and DDD/1,000 inhabitants/day was studied for the four groups of cases using a Poisson model. This analysis covers the period from 2010 to 2016, since PCV13 was included as part of the childhood vaccination schedule in 2010.
The statistical programs used were STATA v. 14, GeoDa, SaTScan™ and Jointpoint Trend Analysis.
Results4,678 cases reported to the Notifiable Disease Surveillance System (EDO) were included. The serotypes of 4,466 cases (95.4%) were isolated and identified at the Regional Public Health Laboratory, 1,936 cases in patients over 59 years of age, 22.1% (427 cases) of them due to erythromycin-resistant serotypes.
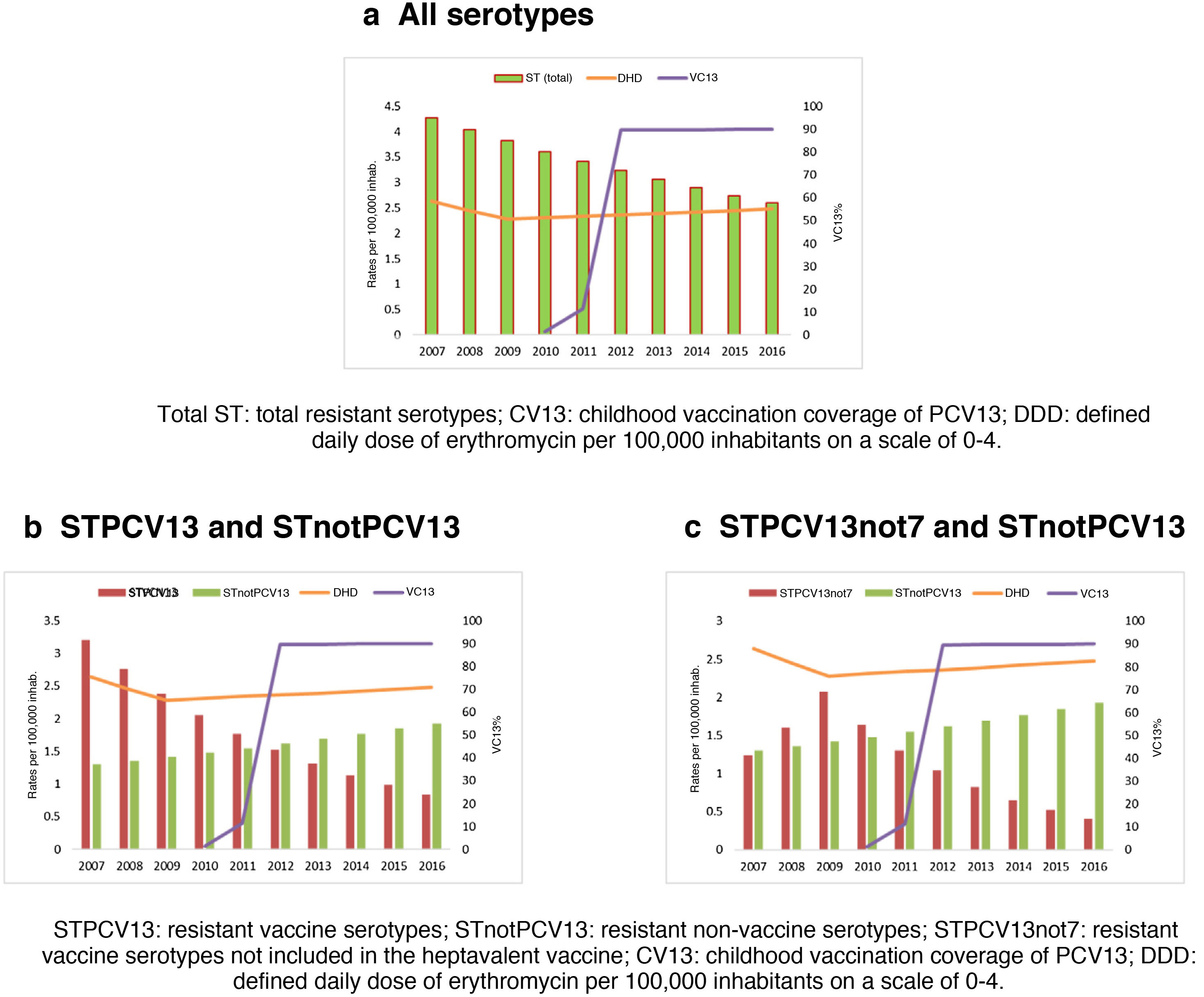
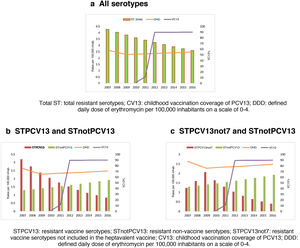
Figs. 2a, 2b and 2c represent the evolution of PCV13 coverage, the consumption of macrolides and the incidence of IPD due to erythromycin-resistant serotypes by serotype group.
The incidence of IPD cases due to erythromycin-resistant serotypes presented a downward trend for all serotypes and for the vaccine serotypes. However, the incidence of cases due to STPCV13not7 increased until 2009, after which it began to decrease, coinciding with the increase in PCV13 coverage. Furthermore, cases due to STnotPCV13 presented an upward trend throughout the period.
Vaccination coverage has been increasing since it was included as part of the childhood schedule in 2010 and peaked in 2012. The consumption of macrolides diminished between 2007 and 2009, remaining stable until the end of the period studied.
Incidence trends were estimated using JoinPoint. Cases due to STPCV13 presented a downward trend throughout the entire period studied (AAPC: −13.8%) and the decrease in cases due to STPCV13not7 was only in the PCV13 period (APC: −20.6%). Both trends were statistically significant. VC13 presented an upward and statistically significant trend as of the year of its inclusion in the childhood vaccination schedule (AAPC: 98.6%). Community consumption of macrolides first presented a period with a downward trend followed by an upward one, both of them statistically insignificant.
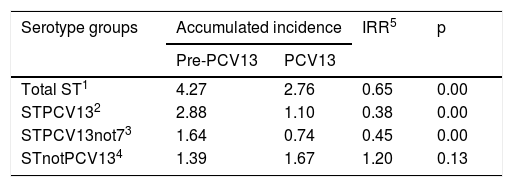
The mean annual incidence for the groups of IPD cases was lower in the PCV13 period, except for cases due to STnotPCV13 (Table 1).
Cumulative incidence of erythromycin-resistant IPD by serotype groups. Comparison between the periods: prePCV13 (2007–2009) and PCV13 (2011–2016).
| Serotype groups | Accumulated incidence | IRR5 | p | |
|---|---|---|---|---|
| Pre-PCV13 | PCV13 | |||
| Total ST1 | 4.27 | 2.76 | 0.65 | 0.00 |
| STPCV132 | 2.88 | 1.10 | 0.38 | 0.00 |
| STPCV13not73 | 1.64 | 0.74 | 0.45 | 0.00 |
| STnotPCV134 | 1.39 | 1.67 | 1.20 | 0.13 |
1: all serotypes; 2: vaccine serotypes; 3: serotypes additional to PCV7; 4: non-vaccine serotypes; 5: Rate ratio index.
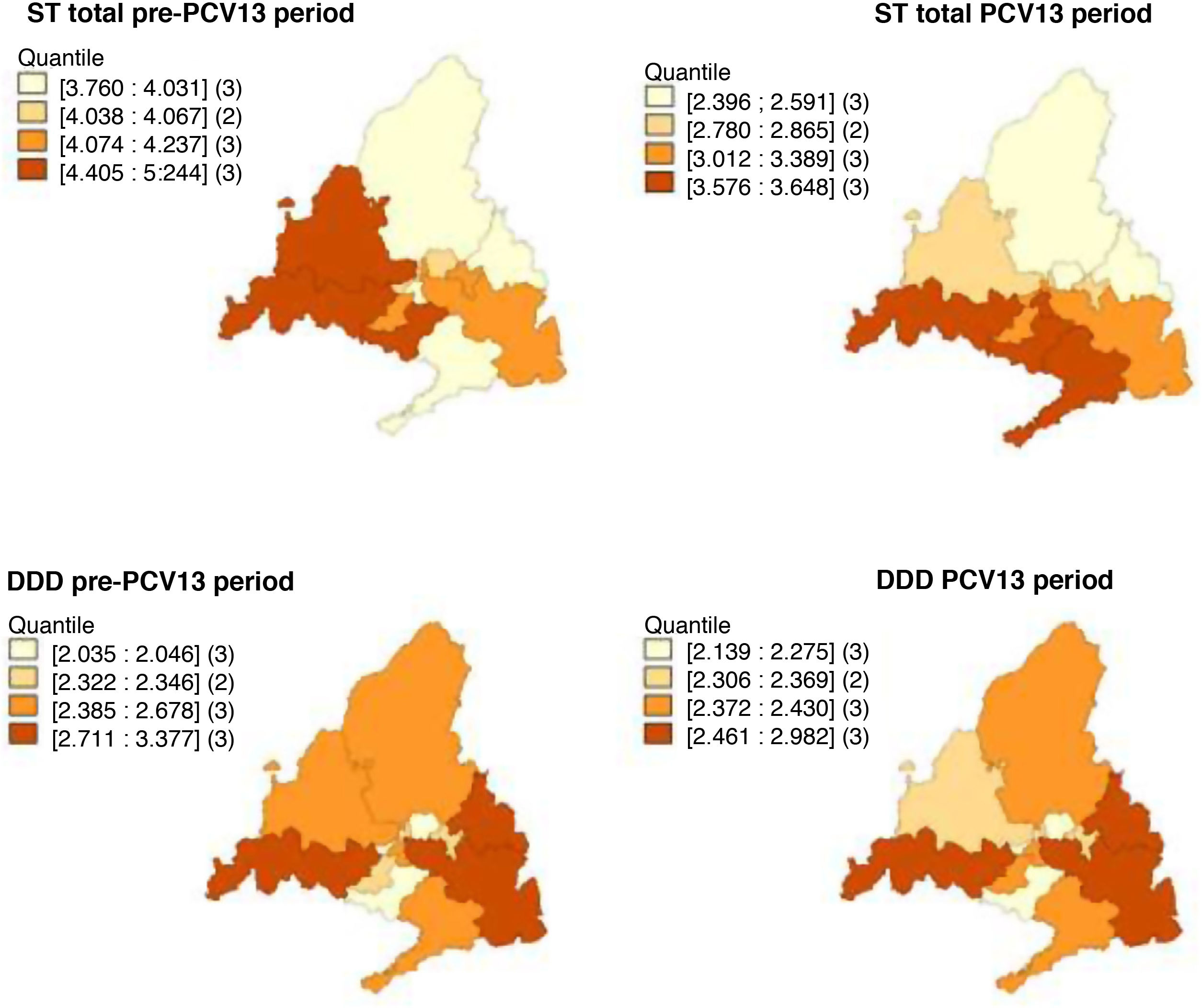
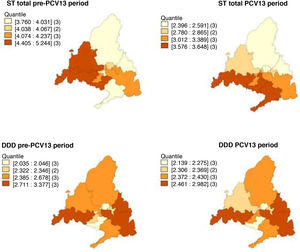
In the pre-PCV13 period, the health areas with the highest smoothed mean incidences of total ST, STPCV13 and STnotPCV13 were 6, 8 and 10, while in the post-PCV13 period it was areas 8, 10 and 1. With regard to the cases due to STPCV13not7, it was areas 6, 1, 8 and 9 in the pre-PCV13 period and 8, 10 and 11 in the PCV13 period (Fig. 3).
For macrolides, the areas with the highest consumption were 1, 3 and 8 in both periods. Consumption increased in area 9 and decreased in area 6 in the PCV13 period (Fig. 3).
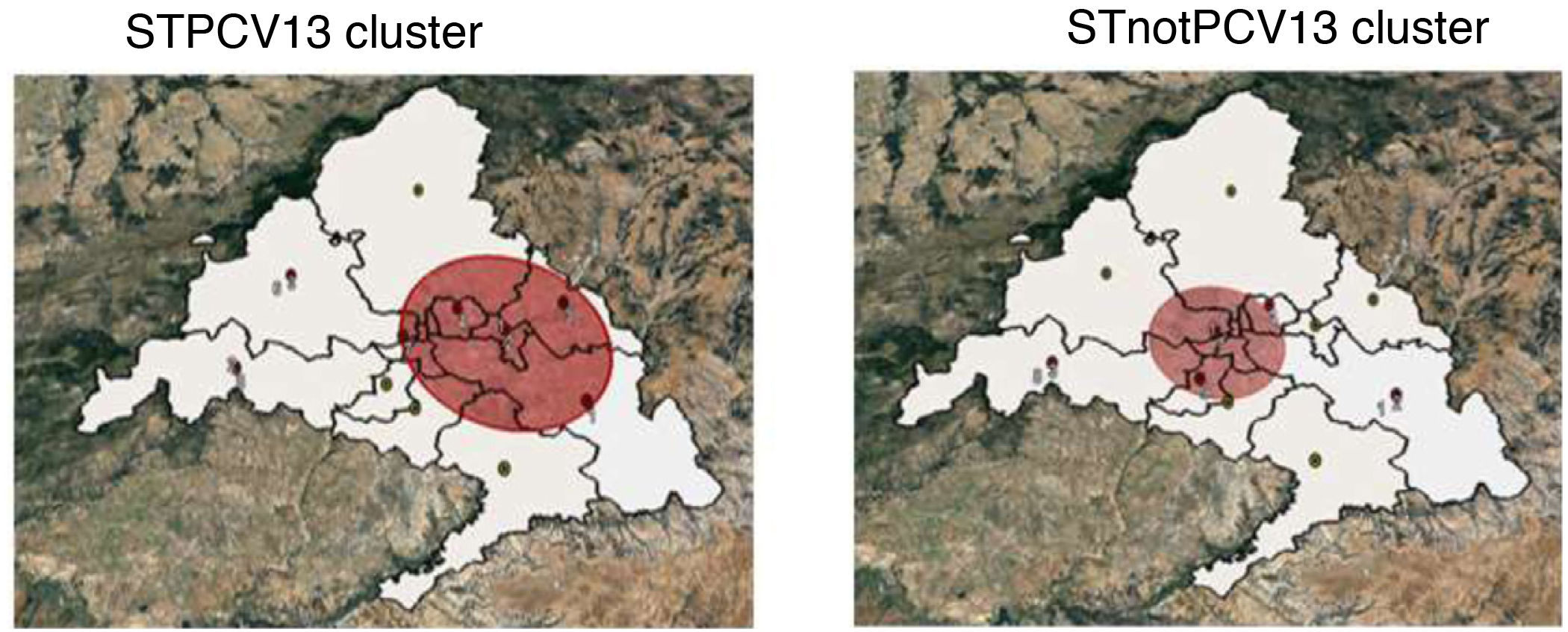
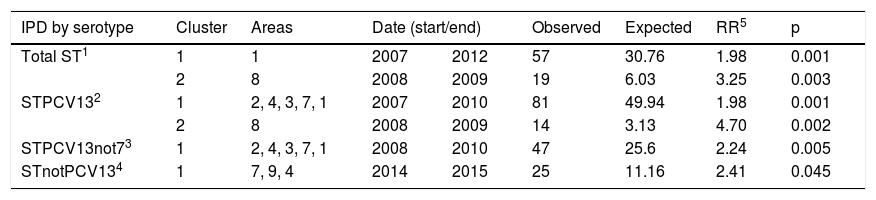
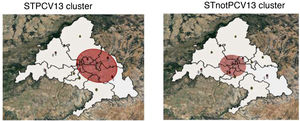
Two clusters were detected due to STPCV13 in the pre-PCV13 periods and one cluster due to STnotPCV13 in the PCV13 period. Health areas 4 and 7 coincided in the clusters due to STPCV13 and STnotPCV13 (Table 2 and Fig. 4).
Spatiotemporal clusters of IPD cases due to erythromycin-resistant serotypes.
| IPD by serotype | Cluster | Areas | Date (start/end) | Observed | Expected | RR5 | p | |
|---|---|---|---|---|---|---|---|---|
| Total ST1 | 1 | 1 | 2007 | 2012 | 57 | 30.76 | 1.98 | 0.001 |
| 2 | 8 | 2008 | 2009 | 19 | 6.03 | 3.25 | 0.003 | |
| STPCV132 | 1 | 2, 4, 3, 7, 1 | 2007 | 2010 | 81 | 49.94 | 1.98 | 0.001 |
| 2 | 8 | 2008 | 2009 | 14 | 3.13 | 4.70 | 0.002 | |
| STPCV13not73 | 1 | 2, 4, 3, 7, 1 | 2008 | 2010 | 47 | 25.6 | 2.24 | 0.005 |
| STnotPCV134 | 1 | 7, 9, 4 | 2014 | 2015 | 25 | 11.16 | 2.41 | 0.045 |
1: total serotypes; 2: vaccine serotypes; 3: serotypes additional to PCV7; 4: non-vaccine serotypes; 5: relative risk.
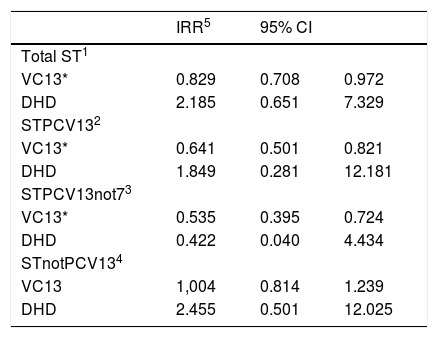
Childhood PCV13 coverage was inversely associated with the incidence of cases due to vaccine serotypes, but not with the incidence of cases due to STnotPCV13. Community macrolide consumption was not associated with the incidence of any of the serotype groups, but the IRR was greater than 2 for the total ST and STnotPCV13 groups (Table 3).
Association of the incidence of IPD due to erythromycin-resistant serotypes with PCV13 childhood coverage and community macrolide consumption. Period 2010–2016.
| IRR5 | 95% CI | ||
|---|---|---|---|
| Total ST1 | |||
| VC13* | 0.829 | 0.708 | 0.972 |
| DHD | 2.185 | 0.651 | 7.329 |
| STPCV132 | |||
| VC13* | 0.641 | 0.501 | 0.821 |
| DHD | 1.849 | 0.281 | 12.181 |
| STPCV13not73 | |||
| VC13* | 0.535 | 0.395 | 0.724 |
| DHD | 0.422 | 0.040 | 4.434 |
| STnotPCV134 | |||
| VC13 | 1,004 | 0.814 | 1.239 |
| DHD | 2.455 | 0.501 | 12.025 |
1: all serotypes; 2: vaccine serotypes; 3: serotypes additional to PCV7; 4: non-vaccine serotypes; 5: Incidence rate ratio.
The incidence of IPD cases due to erythromycin-resistant serotypes included in the PCV decreased in adults above the age of 59 in the period studied, during which time PCV7, and subsequently PCV13, were included in the childhood vaccination schedule. The mechanism of action of PCV consists of reducing the pharyngeal carriage of S. pneumoniae in children under two years old who are considered to be the reservoir and transmitters of the microorganism to the adult population6,7,15. In this way, systematic childhood vaccination reduces the spread of susceptible and resistant vaccine serotypes. This indirect effect of the vaccine has also been observed in neighbouring countries, which have presented reductions in incidence rates in adults16–18. In contrast, in countries with low childhood vaccination coverage, the incidence remained high (Vietnam 72%)19.
In the United States, macrolide-resistant serotypes increased from 18% of isolated cases in 1998 to 45% in 201120. In some European countries, such as Malta or Romania, high prevalences (40%) were also recorded, although in others, such as the Netherlands, prevalence was lower (4.3%)21. In Madrid, the increase in the incidence of cases due to macrolide-resistant vaccine serotypes was observed before the inclusion of PCV13 in the systematic vaccination schedule.
To evaluate the indirect and specific effect of PCV13, we studied the incidence of cases by serotypes included in the vaccine and within them the STPCV13not7, the serotypes targeted by the vaccine. One peculiarity of the evolution of these serotypes was the short increase in their incidence until 2010, when PCV13 was included in the childhood vaccination schedule. This could be due to the pressure created by community macrolide consumption, despite the slight decrease observed in that period, and serotype replacement caused by PCV7 coverage11,22,23. Despite this, our study did not identify significant associations between the incidence of cases and macrolide consumption. This situation could be explained by the stability of macrolide consumption throughout the period studied and the opposite and neutralising effect of childhood vaccination coverage.
The areas with highest macrolide consumption presented a higher incidence of cases. These areas were similar in the pre-PCV13 and PCV13 periods and were located in the south of the CM. According to the MEDEA study, the aforementioned districts group together the basic health zones with the highest rates of deprivation24,25. It is noteworthy that the worsening of socioeconomic conditions is a population risk factor for antibiotic resistance26,27.
Furthermore, the incidence of cases due to non-vaccine serotypes increased in the vaccination period, surpassing the fall in cases due to vaccine serotypes. This behaviour is similar to serotype replacement after the inclusion of PCV728–30. In Western countries with routine childhood vaccination programmes, an increased prevalence of cases due to resistant serotypes not included in the vaccine has also been observed21. Another factor that may account for the increase in cases due to non-vaccine serotypes is related to the dissemination of lineages with mechanisms of resistance to macrolides, as is the case of 24F-CC230 serotypes31.
The spatiotemporal analysis also indicates the possibility of vaccine serotype replacement by non-vaccine serotypes. IPD clusters due to vaccine serotypes were present only in the pre-PCV13 period, indicative of the inhibitory effect of childhood vaccination coverage during the PCV13 period. On the contrary, clusters due to non-vaccine serotypes were reported in the PCV13 period and were regarded as emerging serotypes. Once again, the clusters for both groups of serotypes were identified in areas with a poorer socioeconomic situation.
One of the limitations of the study is the lack of data on childhood vaccination coverage by health area, although it is estimated that there were no significant geographical differences. Another limitation is the fact that socioeconomic variables were not included in the analysis, which could be associated with the distribution of incidence. Furthermore, the nature of the ecological study prevents the results from being extrapolated to the individual level. Despite this, the sources of information used afford the study validity due to the high quality of the data provided by the Notifiable Disease Surveillance System, among which the high proportion of cases with the identified serotype should be noted.
We can conclude that the incidence of IPD due to erythromycin-resistant serotypes has decreased in over-59s at the expense of cases due to STPCV13. At the same time, the increase in the incidence of cases due to non-vaccine serotypes and their detection as a cluster in the PCV13 period indicate vaccine serotype replacement. Finally, community macrolide consumption remained stable throughout the study period and does not appear to have had a decisive bearing on the evolution of cases due to resistant serotypes.
The results obtained support the need to promote vaccination policies and the rational use of antibiotics. The introduction of vaccines that include the most common resistant serotypes could be of great value in controlling IPD caused by them. In any case, it is essential to continue with the active, epidemiological and microbiological surveillance programmes.
FundingNo funding was received for the preparation of this research work.
Conflicts of interestThe authors declare that they have no conflicts of interest.