Some studies indicate high prevalences of latent tuberculosis infection (LTBI) in the immigrant population, which is relevant because 5–10% of cases will develop active tuberculosis. The objective of this study is to describe the results of a sequential strategy in the newly-arrived immigrant population for the diagnosis of LTBI using the tuberculin skin test (TST) and IGRAs.
MethodsA retrospective descriptive study was carried out with immigrants between 6 and 35 years of age from shelters, referred to an international health unit between July 2013 and June 2016. The TST was performed and when it was ≥5mm, IGRAs were conducted. LTBI was defined as an IGRA ≥0.35IU/ml and normal chest X-ray.
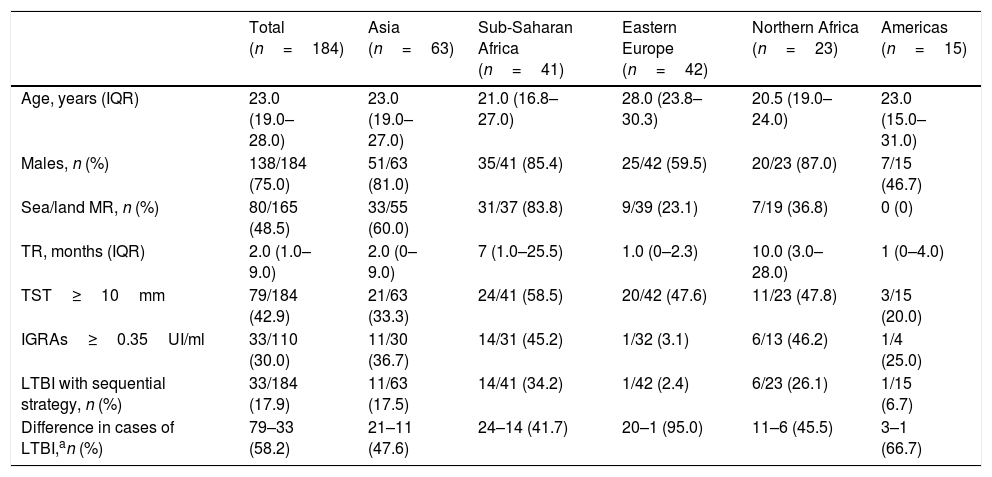
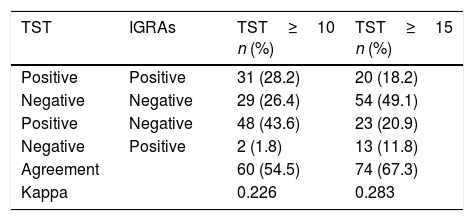
ResultsOf the 184 cases, 138 (75.0%) were men, 23.0 years of age. The most common geographical areas were: 63 (34.2%) from Asia, 42 (22.8%) from Eastern Europe and 41 (22.3%) from sub-Saharan Africa. The TST was ≥10mm in 79 cases (42.9%). The prevalence of LTBI using the sequential strategy was 33/184 (17.9%). Cohen's Kappa index (between TST≥10mm and IGRAs) was 0.226.
ConclusionBasing LTBI screening on the TST alone could give rise to an overestimation. Some studies show that sequential screening would be the most cost-effective; this seems most evident in BCG-vaccinated populations.
Algunos estudios indican altas prevalencias de infección tuberculosa latente (ITBL) en población inmigrante, lo que es relevante, pues el 5-10% de los casos desarrollaran una tuberculosis activa. El objetivo de este estudio es describir los resultados de una estrategia secuencial en población inmigrante recién llegada para el diagnóstico de ITBL usando la prueba de tuberculina (PT) e IGRAs.
MétodosSe realizó un estudio descriptivo retrospectivo con inmigrantes entre 6 y 35años de edad de centros de acogida, derivados a una unidad de salud internacional entre julio de 2013 y junio de 2016. Se realizó la PT, y cuando fue ≥5mm, se determinaron los IGRAs. La ITBL se definió como aquellos con IGRAs ≥0,35UI/ml y radiografía de tórax normal.
ResultadosDe los 184 casos, 138 (75,0%) eran hombres, de 23,0años de edad. Las áreas geográficas de origen más frecuente fueron: 63 (34,2%) de Asia, 42 (22,8%) de Europa del Este y 41 (22,3%) del África subsahariana. La PT fue ≥10mm en 79 (42,9%). La prevalencia de ITBL usando la estrategia secuencial fue de 33/184 (17,9%). El índice kappa de Cohen (entre PT≥10mm e IGRAs) fue de 0,226.
ConclusiónBasar el diagnóstico de la ITBL tan solo en la PT puede representar una sobreestimación. Algunos estudios demuestran que el cribado secuencial sería el más coste-efectivo, y ello parece más evidente en las poblaciones vacunadas con BCG.
According to official data, the number of legal residents in Spain increased from 748,953 in 1999 to 4,424,409 in 2017.1 Some immigrant population studies have presented prevalences of latent tuberculosis infection (LTBI) ranging from 5% to 72% depending on the area of origin or the test used.2 These prevalences tend to be higher than the prevalence seen in the general population. It has been estimated that around 23% of the global population has LTBI. Most cases are in Africa and Asia.3
Early diagnosis of LTBI is important because 5–10% of those with LTBI may develop active tuberculosis.4 This represents not only a problem for individual health, but also a public health challenge. A recently published review found that, in developed countries, screening for LTBI in immigrants from Africa and Asia was cost-effective.3 In addition, given that the prevalence of resistant and multidrug-resistant (MDR) TB is higher in immigrants (16.5 and 9.1%, respectively) compared to the native population (7.6 and 2.8%, respectively), screening seems justified.5 Protocols in immigrants recommend screening for LTBI in those from areas with a high incidence (≥20 cases per 100,000).6 To this end, most guidelines include a tuberculin skin test (TST), a chest X-ray when the TST is positive, and interferon-gamma determination (IGRAs) only in some cases.6
The TST is based on a delayed cell response in which T cells previously exposed to mycobacteria are attracted to the antigen solution injection site and release lymphokines. This results in local induration due to phenomena of vasodilation, oedema and fibrin deposits as well as attraction of other inflammatory cells.7 One limitation of the TST is the fact that it has low specificity, as it contains antigens shared by other mycobacteria. This low specificity may result in false positives in people vaccinated with Bacillus Calmette–Guérin (BCG) and people infected with other mycobacteria.8 It may also result in false negatives in people with abnormal cellular immunity.9 It has the further disadvantage of the need for two medical visits and the potential for variability in reading depending on the reader.
Interferon-gamma release assays (IGRAs) have demonstrated greater specificity than the TST. They are based on the T cell response of an individual with TB to Mycobacterium tuberculosis-specific antigens with the production of specific interferon-gamma (IFN-γ). These are automated; therefore, they are immune to reader bias and subject to less immunological interference (with other mycobacteria or with the BCG vaccine).6 Some laboratories can produce results in 24h; however, this normally takes them a few days. They can also detect a lack of response in anergic patients using internal controls.10 Their disadvantages are their high price and required conditions for sample transport, processing and storage.6 Another challenge is the functioning of both techniques in young children (<5 years of age) due to their immunological immaturity.10
In 2013, a collaborative project started between the Vall d’Hebron-Drassanes Tropical Medicine and International Health Unit (Barcelona, Spain) and social entities (public and private) that welcome immigrants in vulnerable social situations (asylum seekers, unaccompanied minors, vulnerable youth, etc.). This programme included an initial health check-up for anybody who asked, plus health education activities. The objective of this study was to report the results of a sequential strategy for the diagnosis of LTBI (using the tuberculin skin test [TST] and IGRAs) in newly arrived immigrants living in shelters.
MethodologyThis retrospective descriptive study enrolled immigrants between 6 and 35 years of age living in shelters who were referred to the Vall d’Hebron-Drassanes Tropical Medicine and International Health Unit (Barcelona, Spain) between July 2013 and June 2016.
A sequential strategy was used to screen for LTBI. This strategy consisted of the TST in all cases and an IGRAs determination in those with induration ≥5mm. A chest X-ray was ordered in cases of a TST≥5mm or IGRAs≥0.35UI/ml. Two possible LTBI-defining criteria were established: cases of a TST≥10mm and a normal chest X-ray, and cases of IGRAs≥0.35UI/ml and a normal chest X-ray.
The TST was performed by trained nursing staff. It consists of intradermal administration in the flexor surface of the forearm of 0.1ml of a sterile aqueous solution prepared with prolonged culture of human strains of M. tuberculosis. This solution should be stored at 2–8°C. Following injection, a papule measuring around 7mm in diameter should form. The test is examined at 48–72h by measuring the diameter of the indurated area.6
The QuantiFERON-TB Gold In Tube test was used for IGRAs. This test includes M. tuberculosis-specific antigens such as early secretory antigen target (ESAT)-6, culture filtrate protein (CFP)-10 and TB7.7. It comprises 3 tubes: a negative control tube (which contains no reagents) to determine baseline IFN-γ, a positive control tube (which contains phytohaemagglutinin) to confirm baseline immune status and a tube containing M. tuberculosis-specific antigens (ESAT-6, CFP-10 and TB 7.7) to detect CD4+ T cell responses to these antigens. To perform the test, 3ml of blood are drawn, 1ml is injected into each tube and the tubes are shaken. Next, the tubes are incubated for 16–24h in a 37°C oven, then centrifuged. After that, the enzyme immunoassay is prepared with plasma to quantify the IFN-γ released by the patient's lymphocytes. Detection is semi-automatic, and a specific software program is used to issue the results.
Inclusion criteria: immigrants from countries with a high incidence of TB (≥20 cases per 100,000) or immigrants from areas with low incidences of TB but deemed high-risk due to the migration or settlement process, with less than 5 years of residence in Europe and age >5 years and ≤35 years.
Exclusion criteria: individuals who did not meet the inclusion criteria, immunosuppressed individuals, pregnant women, individuals with a positive prior TST and individuals with a history of tuberculous disease.
The variables collected were: sex, date of birth, country of birth, geographical area of origin, date of arrival in Spain, migration route, date of visit, symptoms reported, TST, IGRAs and chest X-ray.
The study was conducted in accordance with the tripartite harmonised standards for good clinical practice as well as the current Spanish national regulations (Law 14/2007 for biomedical studies) and the ethical principles deriving from the Declaration of Helsinki. Study participant data confidentiality will be guaranteed by ensuring compliance with Organic Law 15/1999, of 13 December, on personal data protection. This study was evaluated and approved by the independent ethics committee (IEC) of the Hospital Vall d’Hebron in Barcelona and managed according to good clinical practice guidelines.
For data description we used measures of distribution, central tendency (mean or median if the standard deviation was >20%) and dispersion (interquartile range). Qualitative variables were compared using the chi-squared test or Fisher's exact test for small samples. Continuous variables were compared using Student's t test or the Mann–Whitney U test. A probability value below 0.05 was considered statistically significant. Agreement between the TST and IGRAs was evaluated using Cohen's kappa statistic. This statistic was interpreted as follows: 0–0.2=minimal agreement; 0.2–0.4=limited agreement; 0.4–0.6=moderate agreement; 0.6–0.8=good agreement; 0.8–1=very good agreement. Statistical analysis was performed using the SPSS 23.00® software program.
ResultsOf the 194 patients initially enrolled, 10 were excluded: 6 asymptomatic patients who did not undergo a chest X-ray despite a positive TST (3 with positive IGRAs); 2 men who were carriers of human immunodeficiency virus (HIV); one man who at follow-up visits reported a possible history of tuberculous disease, and one asymptomatic 15-year-old Pakistani male who had been living in Spain for 2 months and was diagnosed with MDR TB (TST 15mm, IGRAs positive and X-ray with right upper lobe pulmonary infiltrate).
Of the 184 cases included, 138 (75.0%) were men, with a median age of 23.0 years (IQR: 19.0–28.0). The most common geographical areas of origin were as follows: 63 (34.2%) were from Asia, 42 (22.8%) were from Eastern Europe, 41 (22.3%) were from sub-Saharan Africa, 23 (12.5%) were from North Africa and 15 (8.2%) were from the Americas (Table 1). Their median time of residence was 2.0 months (IQR: 1.0–9.0). Altogether, 137/184 (74.5%) were asylum seekers. Among them, 80/165 (48.5%) followed a sea/land migration route. In addition, 172/184 (93.5%) were asymptomatic. The TST was <5mm in 74 (40.2%), 5–9mm in 31 (16.9%) and ≥10mm in 79 (42.9%). Of the 110 with a TST≥5mm who underwent an IGRAs determination, the result was positive in 33/110 (30.0%), representing a prevalence of LTBI of 33/184 (17.9%) using the sequential strategy (Table 2). The level of agreement calculated with Cohen's kappa was 0.226 for a TST≥10mm (Table 3).
Area and country of origin.
| Geographic area of origin | Cases (%) | Country of origin | Cases (%) |
|---|---|---|---|
| Asia | 63 (34.2) | Afghanistan | 4 (2.2) |
| Armenia | 1 (0.5) | ||
| Bangladesh | 2 (1.1) | ||
| India | 2 (1.1) | ||
| Jordan | 2 (1.1) | ||
| Nepal | 1 (0.5) | ||
| Pakistan | 22 (12.0) | ||
| Syrian Arab Republic | 17 (9.2) | ||
| Palestine | 7 (3.8) | ||
| Uzbekistan | 5 (2.7) | ||
| Eastern Europe | 42 (22.8) | Russia | 6 (3.3) |
| Ukraine | 36 (19.6) | ||
| Sub-Saharan Africa | 41 (22.3) | Cameroon | 4 (2.2) |
| Côte d’Ivoire | 1 (0.5) | ||
| The Gambia | 7 (3.8) | ||
| Ghana | 3 (1.6) | ||
| Guinea | 2 (1.1) | ||
| Guinea-Bissau | 3 (1.6) | ||
| Mali | 10 (5.4) | ||
| Nigeria | 2 (1.1) | ||
| Central African Republic | 2 (1.1) | ||
| Senegal | 5 (2.7) | ||
| Sierra Leone | 1 (0.5) | ||
| Somalia | 1 (0.5) | ||
| Northern Africa | 23 (12.5) | Algeria | 4 (2.2) |
| Egypt | 4 (2.2) | ||
| Morocco | 15 (8.2) | ||
| Americas | 15 (8.2) | Cuba | 1 (0.5) |
| Chile | 1 (0.5) | ||
| Dominican Republic | 1 (0.5) | ||
| El Salvador | 4 (2.2) | ||
| Honduras | 3 (1.6) | ||
| Mexico | 1 (0.5) | ||
| Panama | 1 (0.5) | ||
| Venezuela | 3 (1.6) | ||
Socio-demographic and prevalence data for LTBI by area of origin.
| Total (n=184) | Asia (n=63) | Sub-Saharan Africa (n=41) | Eastern Europe (n=42) | Northern Africa (n=23) | Americas (n=15) | |
|---|---|---|---|---|---|---|
| Age, years (IQR) | 23.0 (19.0–28.0) | 23.0 (19.0–27.0) | 21.0 (16.8–27.0) | 28.0 (23.8–30.3) | 20.5 (19.0–24.0) | 23.0 (15.0–31.0) |
| Males, n (%) | 138/184 (75.0) | 51/63 (81.0) | 35/41 (85.4) | 25/42 (59.5) | 20/23 (87.0) | 7/15 (46.7) |
| Sea/land MR, n (%) | 80/165 (48.5) | 33/55 (60.0) | 31/37 (83.8) | 9/39 (23.1) | 7/19 (36.8) | 0 (0) |
| TR, months (IQR) | 2.0 (1.0–9.0) | 2.0 (0–9.0) | 7 (1.0–25.5) | 1.0 (0–2.3) | 10.0 (3.0–28.0) | 1 (0–4.0) |
| TST≥10mm | 79/184 (42.9) | 21/63 (33.3) | 24/41 (58.5) | 20/42 (47.6) | 11/23 (47.8) | 3/15 (20.0) |
| IGRAs≥0.35UI/ml | 33/110 (30.0) | 11/30 (36.7) | 14/31 (45.2) | 1/32 (3.1) | 6/13 (46.2) | 1/4 (25.0) |
| LTBI with sequential strategy, n (%) | 33/184 (17.9) | 11/63 (17.5) | 14/41 (34.2) | 1/42 (2.4) | 6/23 (26.1) | 1/15 (6.7) |
| Difference in cases of LTBI,an (%) | 79–33 (58.2) | 21–11 (47.6) | 24–14 (41.7) | 20–1 (95.0) | 11–6 (45.5) | 3–1 (66.7) |
IGRAs: Quantiferon-TB Gold test; LTBI: latent tuberculosis infection; TST: tuberculin skin test; MR: migration route; TR: time of residence.
The study sample mostly consisted of young men from Ukraine, Pakistan, Syria and Morocco. These nationalities do not correspond perfectly to the most common nationalities in Spain, according to the Spanish National Statistics Institute (INE). According to the INE, the most common countries of origin are as follows: Romania, Morocco, United Kingdom, Italy, China and Ecuador.1 Our study population consisted of more socially vulnerable immigrants living in shelters; many of them (75%) were asylum seekers. According to Spanish Commission for Refugees (CEAR) data, the three most common geographical areas of origin of asylum seekers in Spain are the following: Syrian Arab Republic, Ukraine and Palestine.11 In addition, Barcelona has the fifth-largest Pakistani population of any European city. This is one reason why some Pakistanis in pursuit of better living conditions choose to settle there.12
The percentage of males was greater in our sample (75%) than the percentage in the immigrant population indicated by the INE (51%). This is probably because the proportion of males is typically higher in some of the more common nationalities in our study (Pakistani and Malian).1 This percentage is that expected in newly arrived asylum seekers in Europe (45–80% males).13
Prevalence of latent tuberculosis infectionThe percentage of LTBI using exclusively the TST was 43% — higher than that expected in the global population (23%), but similar to that published in other studies with an immigrant population (19–70%).2,3 The prevalence ranged from 20% to 59% by area of origin (sub-Saharan Africa 59%, Northern Africa 48%, Eastern Europe 48%, Asia 33% and Latin America 20%). This, too, did not match the published data (Asia 28–31%, Africa 22% and Eastern Europe 16%).3 Our sample consisted of vulnerable immigrants, many of whom had lived on the street; this might have increased their risk of TB.14 The frequency of LTBI using the study's sequential strategy was 18% (2–34%, by area of origin). This figure fell within the values expected based on similar studies (17–45%).15–18
Agreement and kappaOur study's percentage of agreement (55%) was lower than that of other studies, and our kappa (0.2) indicating low agreement fell within the range found in other publications (0.2–0.6).16,19–21 The prevalence of LTBI decreased by 58% using TST and IGRAs sequentially (from 42% to 95% by area of origin). Similar studies in Spain, Norway and the United States with an immigrant population (mostly Africans and Asians) showed a decrease from 25% to 43%.16,17,21 This decrease in cases of LTBI could be due to various reasons. The first reason is the percentage of BCG vaccination, especially in areas with revaccination policies, such as Eastern Europe (in our study, variation was 95% for cases from this area). However, some studies have not shown that this vaccination has to be a determining factor.16,21,22 The second reason is the area of origin, since in areas where the prevalence of LTBI and TB is higher, tests will show higher levels of agreement, as in cases from sub-Saharan Africa.21 The final reason is erroneous TST readings and IGRAs technique errors.6,7
In any case, the decrease in the prevalence of LTBI in using the sequential strategy is important, since the possible side effects of chemoprophylaxis are non-negligible. In addition, our study population was in a vulnerable social situation as subjects lacked fixed short- and medium-term addresses and therefore would struggle to attend the visits for a treatment such as this. Ultimately, these results support the recommendation of other studies and institutions to use sequential screening with a TST and IGRAs, as it is most cost-effective.21–24 This is a typical screening strategy in which a high-sensitivity test is followed by a higher-specificity test. Another solution would be to base screening exclusively on IGRAs. This would essentially depend on the centre's economic capabilities, as it is a more costly test.
TuberculosisOne case of TB, which would represent 1/185 (0.5%), was excluded. This prevalence of active TB would be similar to that observed in another study in Spain with immigrants in a vulnerable social situation and short times of residence (<1 year).16 However, another study in Spain, in immigrants with longer periods of residence (mean of 29 months), showed a prevalence of 5%.25 According to some sources, the risk of developing TB in this population persists for the first 5–7 years of residence, during which time they supposedly manage to stabilise their lives in the destination country.2,26 Our case was a patient with MDR TB who reported no symptoms at the first visit. This supports the indication to screen for LTBI in this population.
LimitationsFirst, as this as a retrospective study, there were losses in at least one variable: 6 patients who did not undergo a chest X-ray (excluded from the analysis) and 19/184 (10%) who had an unknown migration route. Second, immune response may be different in children versus adults. To minimise this variation, children under 5 years of age were excluded from the study. Third, there were no data on IGRAs results in cases with a TST<5mm (40% of the sample); therefore, our level of agreement could have been underestimated. In the group with a TST=5–9mm, just 2/31 (6.5%) of cases were positive for IGRAs. In other studies with an immigrant population in which a TST and IGRAs were performed in all cases, the level of agreement of negative tests was very high.17,21 Therefore, had we performed the calculations, assuming that 100% of cases with a TST<5 were negative for IGRAs, the kappa would have been 0.4, which would have corresponded to moderate agreement. Fourth, we lacked data on the BCG vaccination status of the study population. The BCG vaccine has existed for more than 80 years and is one of the most commonly used vaccines in the world.27 The WHO recommends its administration in all healthy newborns from countries with a high incidence of tuberculosis, thus achieving levels of coverage of >80% of children in countries where it is included in the vaccination programme.28 It could be assumed that the vast majority of the study population was vaccinated. In any case, recording this piece of information based on individual memory or a scar from the vaccination would also have limitations, since, on the one hand, it would be based on subjective memory in each case and, on the other hand, a scar may be absent in half of cases.29 Finally, there is no gold standard for the diagnosis of LTBI; therefore, we could not affirm that most cases of LTBI using the TST were false positives.
Basing the diagnosis of LTBI on the TST alone could lead to overestimating the actual prevalence of LTBI. This is important given the potential side effects of chemoprophylaxis for LTBI. Using IGRAs could improve this situation due to its greater specificity. However, given the absence of a gold-standard test to make this diagnosis, it cannot be affirmed that most cases using the TST are false positives. Some studies have shown that sequential screening with both techniques would be most cost-effective, given the savings on unnecessary visits and treatments. This seems most obvious in BCG-vaccinated populations.
FundingNo funding.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Serre-Delcor N, Treviño-Maruri B, Tórtola MT, Fernández-Quevedo M, Soriano-Arandes A, Oliveira-Souto I, et al. Estrategia secuencial para el cribado de la ITBL en inmigrantes recién llegados en situación social vulnerable. Enferm Infecc Microbiol Clin. 2018;36:550–554.