Immune response stimulation may be an adjuvant to antimicrobial treatment. Here, we evaluated the impact of immune response modification by lysophosphatidylcholine (LPC), combined with imipenem or ceftazidime, in murine models of peritoneal sepsis (PS) and pneumonia induced by Pseudomonas aeruginosa.
MethodsThe imipenem and ceftazidime-susceptible strain (Pa39) and imipenem and ceftazidime-resistant strain (Pa238) were used. Ceftazidime pharmacokinetic and pharmacodynamic parameters were determined. The therapeutic efficacy and TNF-α and IL-10 levels were determined in murine models of PS and pneumonia induced by Pa39 and Pa238 and treated with LPC, imipenem or ceftazidime, alone or in combination.
ResultsIn the PS model, LPC+ceftazidime reduced spleen and lung Pa238 concentrations (−3.45 and −3.56log10CFU/g; P<0.05) to a greater extent than ceftazidime monotherapy, while LPC+imipenem maintained the imipenem efficacy (−1.66 and −1.45log10CFU/g; P>0.05). In the pneumonia model, LPC+ceftazidime or LPC+imipenem reduced the lung Pa238 concentrations (−2.37log10CFU/g, P=0.1, or −1.35log10CFU/g, P=0.75). For Pa39, no statistically significant difference was observed in the PS and pneumonia models between combined therapy and monotherapy. Moreover, LPC+imipenem and LPC+ceftazidime significantly decreased and increased the TNF-α and IL-10 levels, respectively, in comparison with the untreated controls and monotherapies.
ConclusionsThese results demonstrate the impact of immune response modification by LPC plus antibiotics on the prognosis of infections induced by ceftazidime-resistant P. aeruginosa.
La estimulación de la respuesta inmunitaria podría ser adyuvante al tratamiento antimicrobiano. En este estudio, hemos evaluado el impacto de la modificación de la respuesta inmunitaria por la lisofosfatidilcolina (LPC), combinada con imipenem ó ceftazidima, en modelos murinos de sepsis peritoneal (SP) y de neumonía por Pseudomonas aeruginosa (P. aeruginosa).
MétodosLa cepa sensible a imipenem y ceftazidima (Pa39) y la cepa resistente a ambos antibióticos (Pa238) fueron usadas. Los parámetros farmacocinéticos/farmacodinámicos de ceftazidima fueron determinados. La eficacia terapéutica y los niveles de TNF-α and IL-10 fueron determinados en los modelos murinos de SP y de neumonía por Pa39 y Pa238 y tratados con LPC, imipenem o ceftazidima, en monoterapia ó en combinación.
ResultadosEn el modelo de SP, LPC + ceftazidima redujo la concentración de Pa238 en el bazo y el pulmón (–3,45 y –3,56 log10 UFC/g; p < 0,05) en comparación con ceftazidima, mientras LPC + impenem mantuvo la eficacia de imipenem (–1,66 y –1,45 log10 UFC/g; p > 0,05). En el modelo de neumonía, LPC + ceftazidima o LPC + imipenem redujo la concentración de Pa238 en pulmón (–2,37 log10 UFC/g, p = 0,1 o –1,35 log10 UFC/g, p = 0,75). Para Pa39, no se observó diferencia estadística significativa entre la terapia combinada y la monoterapia en los modelos de SP y de neumonía. Además, LPC + imipenem y LPC + ceftazidime redujeron y aumentaron los niveles de TNF-α y IL-10, respectivamente, en comparación con los controles no tratados y las monoterapias.
ConclusionesEstos resultados demuestran el impacto de la modificación de la respuesta inmunitaria por LPC en combinación con antibióticos en el pronóstico de las infecciones por P. aeruginosa ceftazidima-resistente.
Pseudomonas aeruginosa, a ubiquitous microorganism, is one of the most relevant pathogens causing human opportunistic infections.1P. aeruginosa is a leading cause of severe nosocomial infections, particularly in critically ill and immunocompromised patients.2,3 Indeed, P. aeruginosa is the top pathogen causing ventilator-associated pneumonia and burn wound infections and is a major cause of nosocomial bacteremia.2–4 During the last decade, this pathogen has become increasingly resistant to most antimicrobials, including imipenem and ceftazidim.5
An MDR pattern is commonly observed for P. aeruginosa clinical isolates, raising the threat of difficult-to-treat infections6–8 These MDR isolates are generally susceptible to polymyxins (colistin and polymyxin B) and resistant to imipenem and ceftazidime.9 In a study including bacteremic patients affected by P. aeruginosa, ceftazidime and imipenem resistance rates were 36.6% and 22.8%, respectively, and a multivariate analysis showed that resistance to both antimicrobial agents is a significant factor associated with mortality.10
While new beta-lactamases inhibitors, combined with existing antibiotic families, such as ceftazidime/avibactam, ceftolozane/tazobactam, and imipenem/relebactam, against specific carbapenemases, have recently been developed,11 the retreat of the pharmaceutical sector from new antibiotic development has exacerbated the challenge of widespread resistance and signals a critical need for innovation, for instance, non-antimicrobial approaches and repurposing drugs. All these reasons have made the search for new alternatives to the treatment and control of infections caused by P. aeruginosa urgent and necessary.5,6 Not killing bacteria, but instead avoiding the infection produced by P. aeruginosa, either by immunizing the host or blocking the bacterial virulence factors, could constitute an adjuvant approach to reach new therapeutic goals.
Immune system stimulation by lysophosphatidylcholine (LPC) is one of the most promising approaches. LPC is a major component of the phospholipids. It has been reported to stimulate different immune cells such as monocytes, macrophages, lymphocytes T and neutrophils.12–15 Two specific receptors of LPC (GPR4 and G2A) have been described in neutrophils, lymphocytes and fibroblastes.16–18 Moreover, LPC is able to increase the release of INF-gama, IL-2 and IL-12, and to reduce the release of TNF-alpha and IL-1 beta in a murine disseminate polymicrobial sepsis.16,19 As a result of these events, several studies have showed the involvement of LPC in the recruitment of immune cells,19–21 suggesting its possible role in the elimination of prokaryotic cells during infection.
We have successfully demonstrated the efficacy of LPC at 25mg/kg as a pre-emptive treatment in monotherapy and in combination with colistin, tigecycline or imipenem in experimental models of murine peritoneal sepsis and pneumonia caused by susceptible and MDR Acinetobacter baumannii.22,23 In addition Miyazaki et al. have showed that LPC in combination with gentamycin is active against methicillin-resistant Staphyloccous aureus.24 However, no studies have evaluated the therapeutic efficacy of LPC in combination with ceftazidime and imipenem against P. aeruginosa.
In this study, we aimed: (i) to evaluate the efficacy of LPC in combination with imipenem or ceftazidime in murine models of peritoneal sepsis and pneumonia caused by susceptible (Pa39) and MDR (Pa238) P. aeruginosa strains; and (ii) to determine the impact of immune response modification by LPC in combination with imipenem or ceftazidime in both models of infections caused by both strains.
Materials and methodsBacterial strainsP. aeruginosa (Pa39), a clinical strain susceptible to ceftazidime and imipenem, was isolated from a blood culture, and MDR P. aeruginosa (Pa238), a clinical strain resistant to ceftazidime, imipenem, ciprofloxacin and tobramycin, and harboring the metallo-betalactamase VIM-2, was isolated from a blood culture. Both strains were from the REIPI-GEIH 2008 collection.25
Antimicrobial agents and reagentsFor the in vitro assays, antimicrobials were used as standard laboratory powders: ceftazidime and imipenem (Sigma, Spain). For the in vivo experiments, clinical formulations of antimicrobials were used: ceftazidime (Normon, Spain) and imipenem (Merk Sharp and Dohme, Spain). The anesthetic was 5% (w/v) sodium thiopental, which was administered intraperitoneally (i.p.) (B. Braun Medical S.A., Spain).
In vitro susceptibility testingMinimal inhibitory concentrations (MICs) were determined by broth microdilution assay according to the standard CLSI recommendations,26 which have previously been described.27Escherichia coli ATCC 25922 was used as a control strain.
AnimalsEight-week-old immunocompetent C57BL/6 female mice, weighting 18–20g (Production and Experimentation Animal Center, University of Seville, Seville, Spain), were used. The animals were housed in regulation cages and given free access to food and water. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals.28 The protocol was approved by the Committee of Ethics for Animal Experiments of the University Hospital of Virgen del Rocío of Seville (2014PI/014).
Antimicrobial pharmacokinetics and pharmacodynamics parametersSerum LPC and imipenem levels were previously determined by our research group.22,23 Serum ceftazidime levels were determined in groups of 30 healthy mice, following single doses of 100mg/kg i.p. of ceftazidime. In sets of three animals, blood samples were obtained from anesthetized mice from the periorbital plexus at 0, 5, 10, 15, 30, 60, 90, 120, 240, 480 and 1440min after antimicrobial administration. Concentrations of ceftazidime were measured using HPLC-tandem mass spectrometry (LC-MS/MS).29 The Cmax in serum, AUC0-∞, t1/2, and T>MIC ratios were obtained by a computer-assisted method.30 The final dosing of free ceftazidime in the in vivo experiments was adjusted to achieve a T>MIC of at least ≈40–50% of the dosing interval.31
Experimental murine model of peritoneal sepsisThe previously characterized murine peritoneal sepsis model of A. baumannii was used.22 Briefly, the animals were inoculated i.p. with 0.5mL of the minimal lethal dose of 100 (MLD100) of the Pa39 or Pa238 strains, mixed 1:1 with 10% porcine mucin (Sigma, Spain). The MLD100, lethal dose of 50 (LD50) and lethal dose of 0 (LD0) were determined by inoculating groups of 6 mice i.p. with decreasing concentrations of P. aeruginosa from 7.6 to 3.85 Log CFU/mL for the Pa39 strain, and from 7.8 to 4 Log CFU/mL for the Pa238 strain. The survival of the mice was monitored for 7 days, and these values were determined using the Probit method. LPC therapy was administered as a pretreatment 1h before bacterial inoculation, and antimicrobial therapy was initiated 4h after the bacterial inoculation. Groups of 15 mice were randomly ascribed to the following groups: (1) untreated controls (without treatment); (2) LPC administered once at 75mg/kg i.p. at 1h before bacterial inoculation; (3) ceftazidime administered i.p. at 100mg/kg/12h for 72h; (4) imipenem administered i.m. at 30mg/kg/4h for 24h; and (5) and (6) the combinations of LPC at 75mg/kg and ceftazidime at 100mg/kg/12h, and imipenem at 30mg/kg/4h, respectively. The antimicrobial dosages were chosen after obtaining the PK/PD data.
The mortality was recorded over 24h (for the imipenem treatment groups) or 72h (for the ceftazidime treatment group). After the death or the putting down of the mice at the end of the experimental period, aseptic thoracotomies were performed, and blood samples were obtained by a cardiac puncture for qualitative blood cultures. The samples were inoculated in sterile tubes with 1mL of Luria Bertani (LB) broth and incubated for 24h at 37°C, and then 100μL were plated onto sheep blood agar. The results of the blood cultures are expressed as positive (when ≥1CFU was present in the plate) or negative. The spleen and lungs were aseptically removed and homogenized (Stomacher 80; Tekmar Co., USA) in 2mL of sterile NaCl 0.9% solution. Ten-fold dilutions of the homogenized spleen and lungs were plated onto sheep blood agar for quantitative cultures (Log10 CFU/g of spleen or lung).
Experimental murine model of pneumoniaA previously described experimental murine pneumonia model23 was used to evaluate the efficacy of LPC in monotherapy and in combination with antimicrobial agents against the Pa39 and Pa238 strains. Briefly, the mice were anesthetized by an i.p. injection of 5% (wt/vol) sodium thiopental. They were suspended vertically, and the trachea of each was then cannulated with a blunt-tipped metal needle. The fell of the needle tip against the tracheal cartilage confirmed the intratracheal location. A microliter syringe (Hamilton Co., Reno, NV) was used for the inoculation of 50μL of the MLD100.
The mice remained in a vertical position for 3min and then in a 30° position, until they awoke. The MLD100 and LD0 were determined by inoculating groups of 6 mice intratracheally with decreasing concentrations of the Pa39 and Pa238 strains from 10 to 9Log10CFU/mL and monitoring the survival of the mice for 7 days. The treatment groups were similar to the experimental model of peritoneal sepsis. After the death or putting down of the mice at the end of the experimental period, aseptic thoracotomies were performed, and blood samples for a qualitative blood culture were obtained by a cardiac puncture (data are reported as the number [%] of positive cultures). The lungs were aseptically removed and homogenized as described above for a quantitative culture (data are reported in Log10 CFU/g of the lung).
Cytokine assayBlood samples were collected from the periorbital plexuses of 72 anesthetized mice, which were infected or not infected with Pa238 at the MLD100 in the peritoneal sepsis and pneumonia models and treated or not with imipenem, ceftazidime, the LPC-imipenem combination or the LPC-ceftazidime combination, as previously described.6 The serum levels of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10) were determined in mice at 0, 4, and 8h post-infection or treatment with imipenem or ceftazidime using an enzyme-linked immunosorbent assay (ELISA) (eBioscience, Spain), following the protocol presented in Figure S1.
Statistical analysisDifferences in the bacterial spleen and lung concentrations (mean±standard error of the mean (SEM) log CFU/g of tissue) were assessed by an analysis of variance (ANOVA) and post hoc Dunnett test. Differences in the blood sterility (%) between groups were compared by an χ2 test, after normalization determination by the Kolmogorov–Smirnov test. For the mice survival model, a Kaplan–Meier test was performed to determine the difference between mortality rates. A P-value of <0.05 was considered significant. The SPSS (version 17.0) statistical package was used (SPSS Inc.).
ResultsAntimicrobial susceptibilitiesThe MICs of imipenem, ceftazidime and LPC for the Pa39 strain were 1, 1, and >8000mg/L, respectively. The MICs of imipenem, ceftazidime, and LPC for Pa238 were 32, 64, and >8000mg/L, respectively.
Pharmacokinetic and pharmacodynamic parametersThe pharmacokinetic and pharmacodynamic (PD) data for total imipenem and free ceftazidime are shown in Table S1.
MLD100, LD50, and LD0 of P. aeruginosaTo determine the MLD100, LD50, and LD0 of the Pa39 and Pa238 strains, the murine peritoneal sepsis and pneumonia models were used. In the peritoneal sepsis, the mortality was dependent on the concentration of bacteria in the inoculum (data not shown). The MLD100, LD50, and LD0 of the Pa39 strain were 3.85, 2.57 and <1.81Log10CFU/mL, respectively, and the MLD100, LD50, and LD0 of the Pa238 strain were 6.7, 4.65 and <3.08Log10CFU/mL, respectively. With respect to the murine pneumonia model, the inoculum of both strains was concentrated to 10Log10CFU/mL for each strain to reach 100% of mice mortality. Meanwhile, the LD0 was 9Log10CFU/mL.
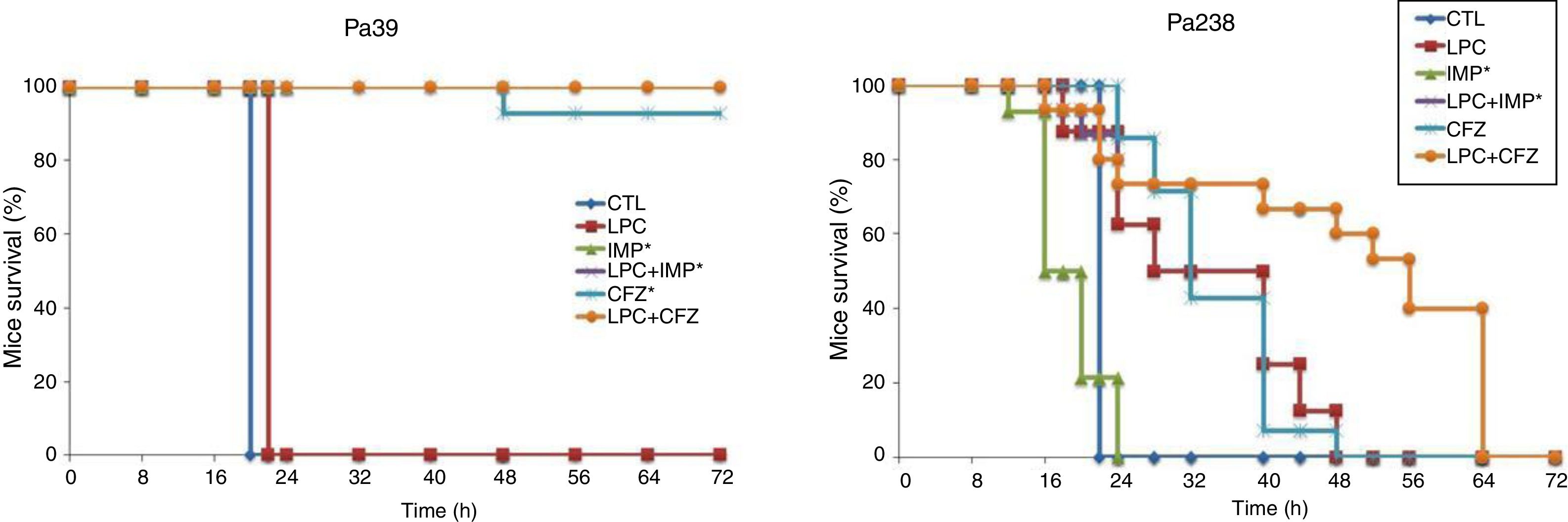
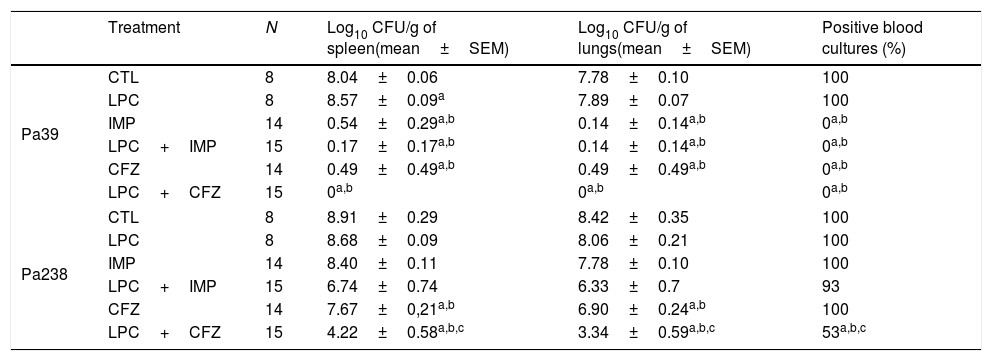
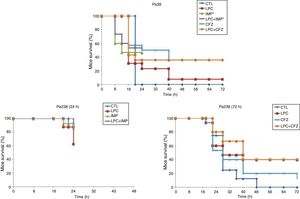
Efficacy of LPC and LPC combined treatments in murine peritoneal sepsis modelThe efficacy of LPC alone and in combination with imipenem or ceftazidime in the murine peritoneal sepsis model, after inoculation with MLD100 of the Pa39 or Pa238 strains is shown in Fig. 1 and Table 1. All treatments, except that with LPC alone, increased the survival rate, compared with the untreated controls, for the Pa39 strain (P<0.05). For the Pa238 strain, only LPC plus imipenem increased mice survival, compared with the untreated controls (P<0.05) (Fig. 1). Regarding the spleen and lung bacterial loads, the imipenem monotherapy decreased the bacterial loads in ≈7.55log10CFU/g (P<0.05), for the Pa39 strain, and in 0.5 and 1.5log10CFU/g, respectively, for the Pa238 strain, compared with the untreated controls. LPC plus imipenem decreased the bacterial loads in both tissues in ≈8log10CFU/g (P<0.05), for the Pa39 strain, and in ≈2log10CFU/g, for the Pa238 strain, compared with the untreated controls or LPC monotherapy. Moreover, ceftazidime monotherapy decreased the spleen and lung bacterial loads in ≈7.5 and ≈7.2log10CFU/g, (P<0.05), respectively, for the Pa39 strain, and in 0.5 and 1.5log10CFU/g (P<0.05), respectively, for the Pa238 strain, compared with the untreated controls. LPC plus ceftazidime decreased the spleen and lung bacterial loads in ≈8log10CFU/g (P<0.05), for the Pa39 strain, and in ≈4.5 and ≈5log10CFU/g (P<0.05), respectively, for the Pa238 strain, compared with the untreated controls or LPC monotherapy (Table 1). With respect to bacteremia induced by the Pa39 strain, the imipenem and ceftazidime monotherapies reduced it to 0%, compared with the untreated controls. For the Pa238 strain, only LPC plus imipenem and LPC plus ceftazidime reduced the bacteremia to 93.33% and 53.33% (P<0.05), respectively, compared with the untreated controls (Table 1).
Mice survival after treatment with LPC in combination with imipenem or ceftazidime in the model of peritoneal sepsis induced by the P. aeruginosa Pa39 and Pa238 strains. CTL, control (no treatment); LPC, lysophosphatidylcholine; IMP, imipenem; CFZ, ceftazidime.*: mice mortality was recorded over 24h in imipenem w/o LPC.
Therapeutic effect of LPC in combination with imipenem or ceftazidime in a murine peritoneal sepsis model of P. aeruginosa.
| Treatment | N | Log10 CFU/g of spleen(mean±SEM) | Log10 CFU/g of lungs(mean±SEM) | Positive blood cultures (%) | |
|---|---|---|---|---|---|
| Pa39 | CTL | 8 | 8.04±0.06 | 7.78±0.10 | 100 |
| LPC | 8 | 8.57±0.09a | 7.89±0.07 | 100 | |
| IMP | 14 | 0.54±0.29a,b | 0.14±0.14a,b | 0a,b | |
| LPC+IMP | 15 | 0.17±0.17a,b | 0.14±0.14a,b | 0a,b | |
| CFZ | 14 | 0.49±0.49a,b | 0.49±0.49a,b | 0a,b | |
| LPC+CFZ | 15 | 0a,b | 0a,b | 0a,b | |
| Pa238 | CTL | 8 | 8.91±0.29 | 8.42±0.35 | 100 |
| LPC | 8 | 8.68±0.09 | 8.06±0.21 | 100 | |
| IMP | 14 | 8.40±0.11 | 7.78±0.10 | 100 | |
| LPC+IMP | 15 | 6.74±0.74 | 6.33±0.7 | 93 | |
| CFZ | 14 | 7.67±0,21a,b | 6.90±0.24a,b | 100 | |
| LPC+CFZ | 15 | 4.22±0.58a,b,c | 3.34±0.59a,b,c | 53a,b,c | |
CTL, untreated controls (no treatment); LPC, lysophosphatidylcholine; IMP, imipenem; CFZ, ceftazidime.
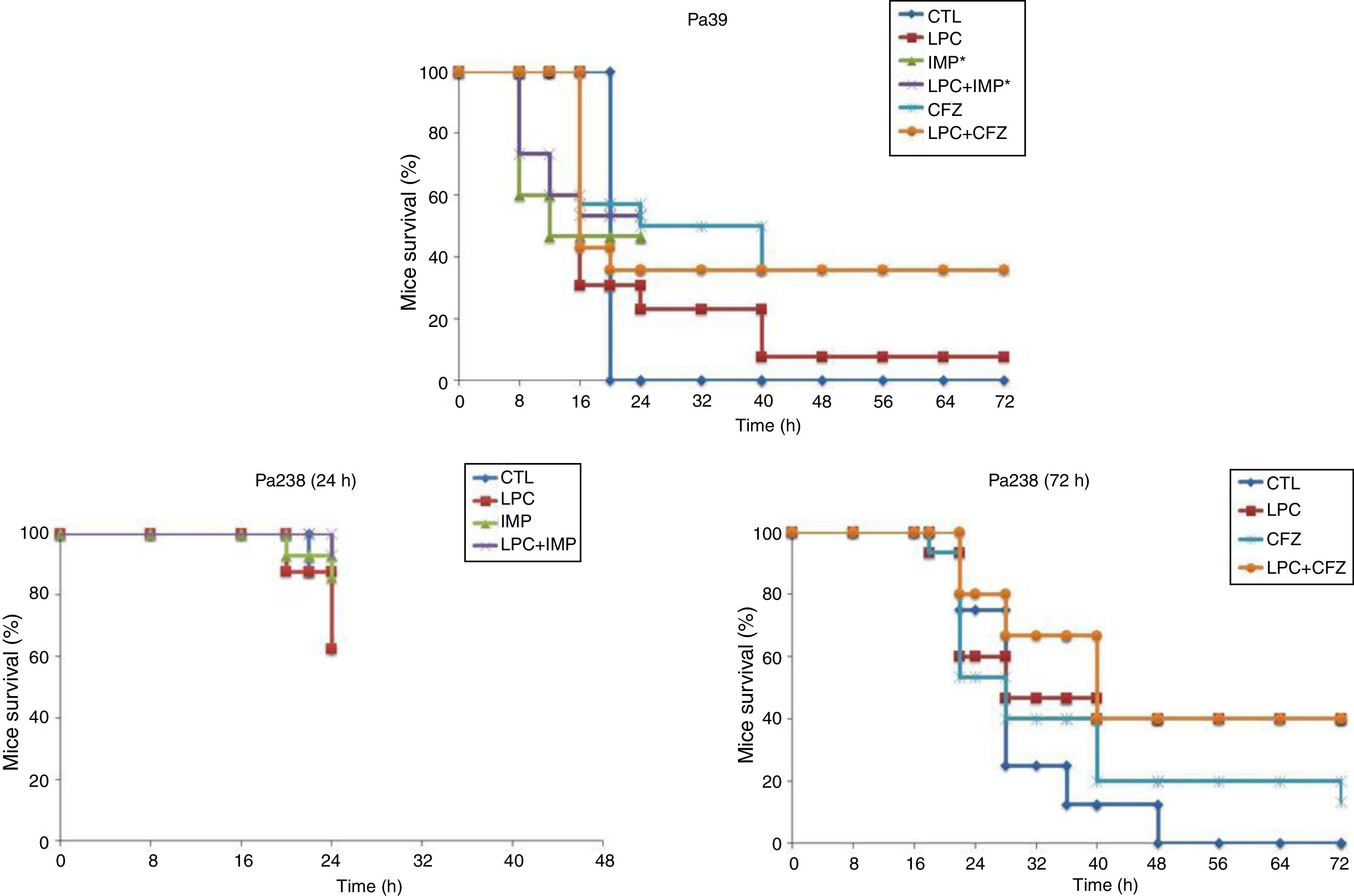
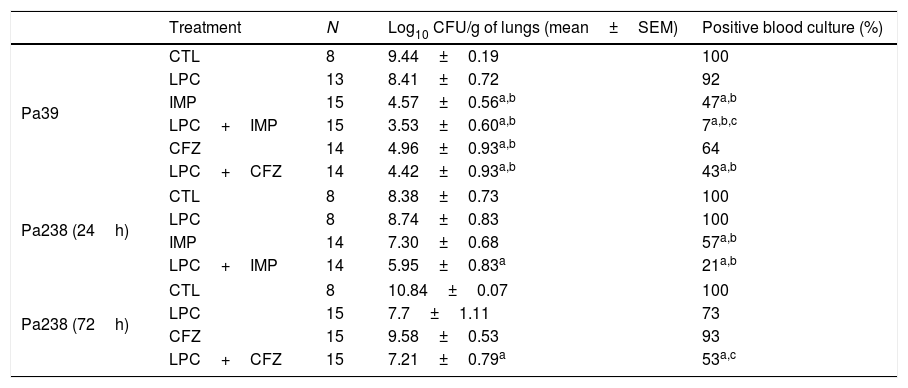
The efficacy of LPC alone and in combination with imipenem or ceftazidime was tested in the murine pneumonia model after an inoculation of 10Log10CFU/mL (MLD100) of each strain (Fig. 2 and Table 2). For both strains, treatment with LPC plus imipenem or LPC plus ceftazidime reduced mortality to 7–64%, compared with the untreated controls (Fig. 2). Regarding the bacterial lung load, imipenem or ceftazidime decreased the Pa39 and Pa238 strains by 4.87 (P<0.05) and 1.08log10CFU/g or 4.48 (P<0.05) and 1.26log10CFU/g, respectively, compared with the untreated controls. LPC plus imipenem decreased the bacterial lung load of the Pa39 and Pa238 strains by 5.91 and 4.89log10CFU/g (P<0.05), or 4.88 (P<0.05) and 2.79log10CFU/g, respectively, compared with the untreated controls or LPC monotherapy. LPC plus ceftazidime decreased the bacterial lung load of the Pa39 and Pa238 strains by 5.02 (P<0.05) and 3.63log10CFU/g, or in 3.99 (P<0.05) and 0.49log10CFU/g, respectively, compared with the untreated controls or LPC monotherapy (Table 2). With respect to bacteremia, LPC plus imipenem and LPC plus ceftazidime reduced both strains of it to ≈50–93%, compared with the untreated controls, and to ≈20–40%, compared with the antimicrobial monotherapies (P<0.05) (Table 2).
Mice survival after treatment with LPC in combination with imipenem or ceftazidime in a model of pneumonia induced by the P. aeruginosa Pa39 and Pa238 strains. CTL, control (no treatment); LPC, lysophosphatidylcholine; IMP, imipenem; CFZ, ceftazidime.*: mice mortality was recorded over 24 h in imipenem w/o LPC.
Therapeutic effect of LPC in combination with imipenem or ceftazidime in a murine pneumonia model of P. aeruginosa.
| Treatment | N | Log10 CFU/g of lungs (mean±SEM) | Positive blood culture (%) | |
|---|---|---|---|---|
| Pa39 | CTL | 8 | 9.44±0.19 | 100 |
| LPC | 13 | 8.41±0.72 | 92 | |
| IMP | 15 | 4.57±0.56a,b | 47a,b | |
| LPC+IMP | 15 | 3.53±0.60a,b | 7a,b,c | |
| CFZ | 14 | 4.96±0.93a,b | 64 | |
| LPC+CFZ | 14 | 4.42±0.93a,b | 43a,b | |
| Pa238 (24h) | CTL | 8 | 8.38±0.73 | 100 |
| LPC | 8 | 8.74±0.83 | 100 | |
| IMP | 14 | 7.30±0.68 | 57a,b | |
| LPC+IMP | 14 | 5.95±0.83a | 21a,b | |
| Pa238 (72h) | CTL | 8 | 10.84±0.07 | 100 |
| LPC | 15 | 7.7±1.11 | 73 | |
| CFZ | 15 | 9.58±0.53 | 93 | |
| LPC+CFZ | 15 | 7.21±0.79a | 53a,c | |
CTL, untreated controls (no treatment); LPC, lysophosphatidylcholine; IMP, imipenem; CFZ, ceftazidime.
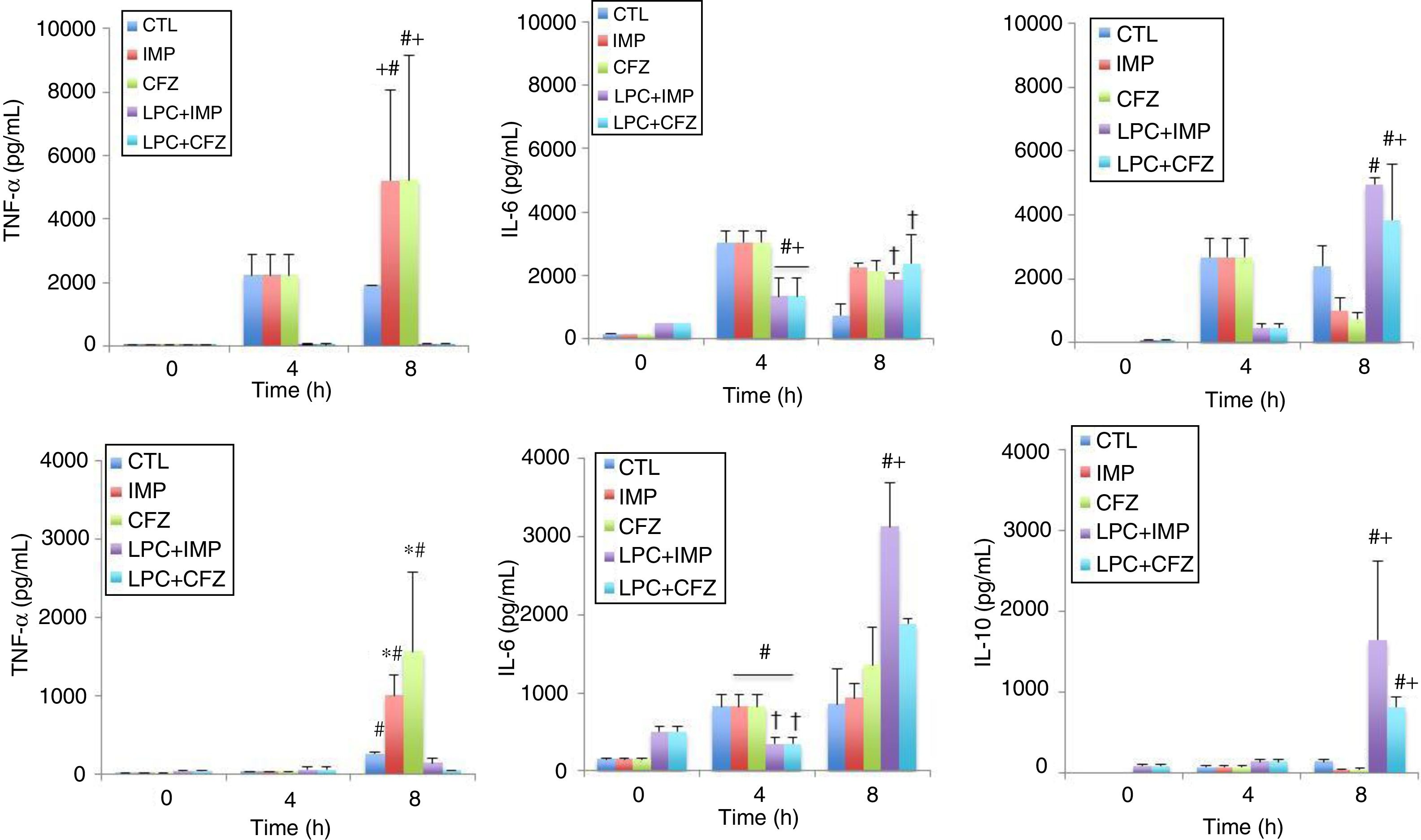
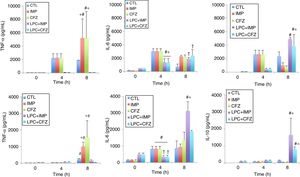
The effects of different treatments on cytokine production in models of peritoneal sepsis and pneumonia induced by the Pa238 strain were evaluated (Fig. 3). In the peritoneal sepsis model, the imipenem and ceftazidime monotherapies non-significantly increased the release of TNF-α to 5207.61±2859.34pg/mL and 5232.59±3905.02pg/mL, respectively, and decreased the release of IL-10 to 1002.25±405.43pg/mL (P<0.05) and 761.96±182.09pg/mL (P<0.05), respectively, at 8h (time that corresponds to 4h of imipenem and ceftazidime treatment), compared with the untreated controls at 8h: 1900.60±638.01pg/mL for TNF-α, and 2423.29±607.45pg/mL for IL-10. Meanwhile, LPC plus imipenem and LPC plus ceftazidime combinations decreased the release of TNF-α to 62.7±14.3pg/mL (P<0.05) and 63.53±19.42pg/mL (P<0.05), respectively, and increased the release of IL-10 to 4950.25±202.67pg/mL (P<0.05) and 3829.5±1760.76pg/mL (P<0.05), respectively, at 8h, compared with the imipenem and ceftazidime monotherapies: 5207.61±2859.34pg/mL and 5232.59±3905.02pg/mL, respectively, for TNF-α, and 1002.25±405.43pg/mL and 761.96±182.09pg/mL for IL-10, respectively. In the case of IL-6, imipenem and ceftazidime monotherapies increased the release of IL-6 to 2274.4±113.51pg/mL (P=0.018) and 2148.58±3.4.03pg/mL (P=0.053), respectively, at 8h, compared with the control group: 748.2±362.84pg/mL. Meanwhile, LPC plus imipenem and LPC plus ceftazidime combinations induced a similar release of IL-6, compared with the imipenem and ceftazidime monotherapies (Fig. 3A).
Experimental models of the cytokine production after MDR P. aeruginosa-induced murine peritoneal sepsis (A) and pneumonia (B). The levels of TNF-α, IL-6 and IL-10 in serum were determined from 0 to 8 h for mice inoculated with the Pa238 strain and treated or not trated with imipenem, ceftazidime, the LPC-imipenem combination or the LPC-ceftazidime combination. Representative results are shown, and the data are presented as the means. CTL, untreated controls (no treatment); LPC, lysophosphatidylcholine; IMP, imipenem; CFZ, ceftazidime. * and †: compared to CTL, P<0.05; #: compared to IMP or CFZ, P<0.05.
In the pneumonia model, similar results concerning the effect of the imipenem and ceftazidime monotherapies and the LPC plus imipenem and LPC plus ceftazidime combinations on the serum TNF-α and IL-10 levels at 8h post-bacterial inoculation were observed. The LPC plus imipenem and LPC plus ceftazidime combinations decreased the release of TNF-α to 150.73±52.7pg/mL (P<0.05) and 54.77±3.21pg/mL (P<0.05), respectively, and increased the release of IL-10 to 1648±969.97pg/mL (P<0.05) and 809.54±130.07pg/mL (P<0.05), respectively, compared with the imipenem and ceftazidime monotherapies: 1007.17±267.19pg/mL and 1564.55±101.84pg/mL, respectively, for TNF-α, and 35.33±14.33pg/mL and 44.75±7.7pg/mL, respectively, for IL-10. In the case of IL-6, the imipenem and ceftazidime monotherapies non-significantly increased the release of IL-6 to 929.42±188.11pg/mL and 1360.49±475.24pg/mL, respectively, at 8h, compared with the control group: 855.40±459.13pg/mL. Moreover, the LPC+imipenem and LPC+ceftazidime combinations increased the release of IL-6 to 3130.40±558.14pg/mL (P=0.043) and 1884.62±68.13pg/mL (P=0.212), respectively, at 8h, compared with the imipenem and ceftazidime monotherapies: 929.42±188.11pg/mL and 1360.49±475.24pg/mL, respectively (Fig. 3B).
Importantly, the imipenem and ceftazidime treatments in healthy mice did not significantly change the release of TNF-α, IL-6, and IL-10at 4 and 8h, when compared with mice that did not receive antibiotic treatments (Fig. 3).
DiscussionAs expected, in the pneumonia model of the MDR strain, no significant increase of mice survival and decrease of bacterial burden in tissues were observed with imipenem or ceftazidime due to resistance to both antimicrobials; however, the treatment with LPC plus imipenem or LPC plus ceftazidime reduced the bacterial loads and bacteremia by ≈1.35–2.35log10CFU/g and 50%, respectively, compared with the antimicrobial monotherapies, and survival increased slightly. Similar data have been observed with LPC plus imipenem or LPC plus tigecycline in a model of A. baumannii resistant to imipenem and tigecycline pneumonia.23
In a peritoneal sepsis model of the MDR Pa238 strain, LPC plus ceftazidime did not improve survival at 72h, even if the bacterial burden in tissue was lower than in untreated controls and the ceftazidime monotherapy. The analysis of survival at 24h showed a mortality of 27% (4 out of 15 mice), a mortality similar to the 20% at 24h with LPC plus imipenem. These data suggest that LPC, administered in one dose before the inoculation, only reduced early mice mortality. In keeping with this result, in 2013, Jacqueline et al. demonstrated that in a murine experimental model of pneumonia induced by P. aeruginosa, the bacterial burden in spleen and lung, after treatment with ceftazidime, was 2.74 and 4.74Log10CFU/mL, respectively, and the mortality reached 80%.32
It is important to note that ceftazidime in combination with LPC against the MDR Pa238 strain in a peritoneal sepsis model did not improve mice survival to a greater extent than in the ceftazidime and LPC monotherapies or control animals. Meanwhile, ceftazidime combined with LPC increased the mice survival in the pneumonia model. In the case of imipenem, the combination with LPC against MDR Pa238 strain has less improved the mice mortality in pneumonia model than in peritoneal sepsis model, according with the results with imipenem monotherapy, which reached 83% in the pneumonia model vs. 0% in the peritoneal sepsis model. Evidence supports this data, in which we have demonstrated previously that imipenem in combination with LPC against MDR A. baumannii Ab186 strain, resistant to imipenem, has improved the mice survival until 100% in the pneumonia model vs. 33.33% in the peritoneal sepsis model, also in according with the survival with imipenem monotherapy (100% vs. 0%, respectively).23 As for the A. baumannii ATCC 17978 strain, we found that the LPC monotherapy in the peritoneal sepsis model only increased 40% of mice survival vs. the 68% observed in the pneumonia model.22 In the same way, rifampicin combined with colistin improved the mice survival in the model of peritoneal sepsis induced by carbapenemase-producing Klebsiella pneumoniae to a lesser extent than in the pneumonia model (unpublished data).
Furthermore, we showed that LPC monotherapy in the model of peritoneal sepsis induced by the susceptible Pa39 strain and MDR Pa238 strain had no significantly increased mice survival and decreased bacterial burden in tissues. In contrast, in the model of pneumonia induced by the susceptible Pa39 and MDR Pa238 strains, we observed that LPC monotherapy for 72h reduced the bacterial loads in the lungs by 1.03 and 3.14log10CFU/g, respectively. This difference in the results between both experimental models of infections is due to the severity of the peritoneal sepsis model, in which the sepsis was defined as the result of a dysregulated systemic inflammatory response syndrome in the presence of infection, accompanied by major organ failure and death.33 This infection severity does not allow LPC in monotherapy to significantly reduce the bacterial loads of both strains in tissues. Moreover, the difference in the effect of LPC on both strains in the pneumonia model has been observed, and this was more present in the case of the MDR Pa238 strain than in the case of the susceptible Pa39 strain. This is due to the difference in the virulence degree of both strains. In the pneumonia model, the susceptible Pa39 strain caused 100% of mice mortality in the first 24h, in contrast with the MDR strain, which caused only 37% of mice mortality in the first 24h. We can suggest that in murine models of P. aeruginosa, it is necessary to administer a LPC dose higher than 25mg/kg to significantly improve survival.
For other pathogens, such as Staphylococcus aureus, Miyazaki et al. showed, in vitro, that LPC can enhance the antimicrobial effects of gentamicin against methicillin-resistant S. aureus (MRSA), suggesting the application of LPC as a beneficial additive to topical antibiotics for superficial skin infections.24 The mode of action of LPC is different, depending on the pathogen species. In Gram positive bacteria, LPC can directly induce MRSA killing by interacting with cytoplasmic membranes, inducing membrane depolarization and increasing membrane permeability.24 In Gram negative bacteria, LPC did not directly affect these bacteria due to their outer membrane, which prevents the interaction between the LPC and bacterial cytoplasmic membrane.16,22,23 The beneficial effects of LPC alone against E. coli have been associated with the activation of hydrogen peroxide by neutrophils, and with the induction of phagocytosis by macrophages through the activation of AMP-activated protein Kinase.16,34 In LPC combined with antibiotic treatments against P. aeruginosa infections, these pathways can be suggested as some of the modes of action of LPC as an adjunct to the antibiotic effect. Besides, LPC alone and in combination with antibiotic treatment against E. coli and A. baumannii have previously been associated with the modulation of inflammation, such as the upregulation of monocyte chemotactic protein-1 and pro- and anti-inflammatory cytokines release.12,16,22,23 Interestingly, comparing the effect of the pro-inflammatory cytokine, TNF-α, with that of the combination of LPC plus imipenem or LPC plus ceftazidime, at 8h post-bacterial inoculation, the treatments significantly reduced the TNF-α levels by 83- or 82-fold, respectively, in the model of peritoneal sepsis induced by the MDR strain. In contrast, these reductions were lower in the pneumonia model: 7- or 28-fold with LPC plus imipenem or LPC plus ceftazidime, respectively. These differences in the anti-inflammatory effect of LPC in both models could be the cause of the different results in terms of mice survival. These data are in accordance with the previously reported immunomodulatory effects of LPC.16,22 It is important to mention that the immune response, developed in mice treated with LPC and ceftazidime and infected by the Pa39 strain in the peritoneal sepsis model, can help to prevent re-infection by the same strain 7 days after the end of the treatment (data not shown). These data allowed us to suggest that LPC in combination with antibiotics should be able to induce immune response memory to prevent reinfection. More studies are needed to decipher this effect.
Some studies have already been performed to control infections caused by P. aeruginosa using small peptides or molecules with immunomodulatory properties. Among them, [E6k,D9k] hymenochirin-1B35 presented high antibacterial activities and immunomodulatory properties in vitro. LL-37, a cationic peptide of the cathelecidins family, exhibited significant antimicrobial activity against P. aeruginosa.36
As demonstrated in this study, LPC, both as a preemptive therapy or in combination with antimicrobial agents, has shown promising in vivo results in severe experimental models of infections induced by A. baumannii22,23 and P. aeruginosa. It is worth noting that the model used in this study was designed to prevent the establishment of infection, following bacterial inoculum. However, caution is needed, and further extensive in vivo studies have to be performed to confirm the potential use of these adjuvants, including LPC, as true therapeutic alternatives. The present study has some limitations regarding the LPC treatments regimens. We believe that the next steps in this research are: (i) to determine whether multiple doses of LPC, given as a treatment in combination with antimicrobial agents, following the establishment of an infection induced by P. aeruginosa, can improve the preemptive effect of LPC; (ii) to evaluate the therapeutic efficacy of LPC in combination with antibiotics against other clinical isolates of P. aeruginosa; and (iii) to use BALB/c mice as another animal model, since BALB/c mice are Th2-biased, while C57BL/C6 mice are Th1-biased. Completing these steps are required in order to consider this adjuvant treatment in future clinical trials.
FundingThis study was supported by the Instituto de Salud Carlos III, Proyectos de Investigación en Salud (grant PI13/01744, PI16/01306), and by Consejería de Innovación, Ciencia y Empresa (P11-CTS-6317).
Younes Smani is supported by the Subprograma Miguel Servet Tipo I from the Ministerio de Economía y Competitividad of Spain (CP15/01358). The MEDINA authors disclosed the receipt of financial support from Fundación MEDINA, a public-private partnership of Merck Sharp & Dohme de España S.A./Universidad de Granada/Junta de Andalucía.
Conflict of interestsThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Antonio Oliver for the kind gift of the Pseudomonas aeruginosa strains Pa39 and Pa238.
Part of this study was presented at the 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria 2017.