Type 1 gastric carcinoids are tumors caused by the constant stimulation of hypergastrinemia on enterochromaffin-like cells occurring in chronic atrophic gastritis. They are the most common gastric carcinoids (70%) and have a good prognosis in most cases. Their indolent course is confirmed by the presence of a low number of mitoses, as measured by the Ki67 index, usually less than 30 per high magnification field, and by a 0–10% risk of angioinvasion or submucosal invasion.1
Metastases occur in 2–5% of cases, usually in regional lymph nodes or liver. Type 1 gastric carcinoids are located in the gastric body or fundus, are usually less than 2cm in size, and are often multiple. Because of their association with chronic atrophic gastritis, the patients most commonly affected are women over 50 years of age, and tumors are incidentally found in gastroscopies performed for anemia or dyspepsia, which are among the symptoms of chronic atrophic gastritis.
Type 1 gastric carcinoids develop following a carcinogenesis model where hypergastrinemia initially causes hyperplasia of enterochromaffin-like cells, after which neoplasms are formed following a period of cell dysplasia.2 The presence of cell dysplasia involves a 26-fold greater risk for the subsequent formation of carcinoid tumors as compared to chronic atrophic gastritis without dysplasia.3 Molecular markers reported in the literature include elevation in most patients of V-MAT2, chromogranin A or synaptophysin, and occasionally histamine. Such elevations are quite non-specific as compared to other gastric neuroendocrine tumors.4
Because of the indolent course of type 1 gastric carcinoids, the therapeutic approach is less aggressive than for other gastric exocrine tumors. In addition, optimal treatment and monitoring of patients are controversial because of the rarity of the disease, and no large, randomized clinical trials which could allow us to draw clear conclusions are available. Endoscopic resection is usually performed when lesion size and number allow. However, the recurrence rate is high. Antrectomy has been performed to eliminate the source of hypergastrinemia, which is essential in the pathophysiology of the disorder.5 More aggressive surgical procedures, including partial or total gastrectomy, have been performed on tumors of a bigger size or with submucosal invasion or angioinvasion.6 Somatostatin analogues may also be used as an effective treatment for gastric carcinoids because they control hypergastrinemia and subsequent hyperplasia of enterochromaffin-like cells.7,8 Some authors would advocate their use for “functioning” gastric carcinoids and in cases with more than six polypoid lesions less than one centimeter in diameter. Three cases of gastric carcinoids are reported below, including their medical treatment and subsequent course. None of the patients were being treated with proton pump inhibitors at diagnosis or during follow-up.
Case 1The case of a 48-year-old female patient with a personal history of primary hypothyroidism of autoimmune origin since she was 30, alopecia universalis, vitiligo, and menopause at 35 years is reported. Routine laboratory tests incidentally found vitamin B12 levels of 24pg/mL (No.>200pg/mL), with folic acid levels within the normal range. Gastrin levels were markedly elevated, 1023pg/mL (No.<108pg/mL), and chromogranin A levels were 6nmol/L (No.<4nmol/L). Autoimmune testing showed positive anti-parietal cell and anti-intrinsic factor antibodies. A gastroscopy was consistent with chronic atrophic gastritis. Two polyps one centimeter in diameter were also seen. An endoscopic ultrasound examination performed to better characterize the findings showed no submucosal invasion of the neoplasms. Endoscopic polypectomy was performed, during which multiple biopsy samples were taken along the gastric cavity. Findings were consistent with chronic atrophic gastritis. Helicobacter pylori was not found. Polyps were reported by the pathological laboratory to be consistent with well differentiated neuroendocrine tumors with no angioinvasion and a Ki 67<2%.
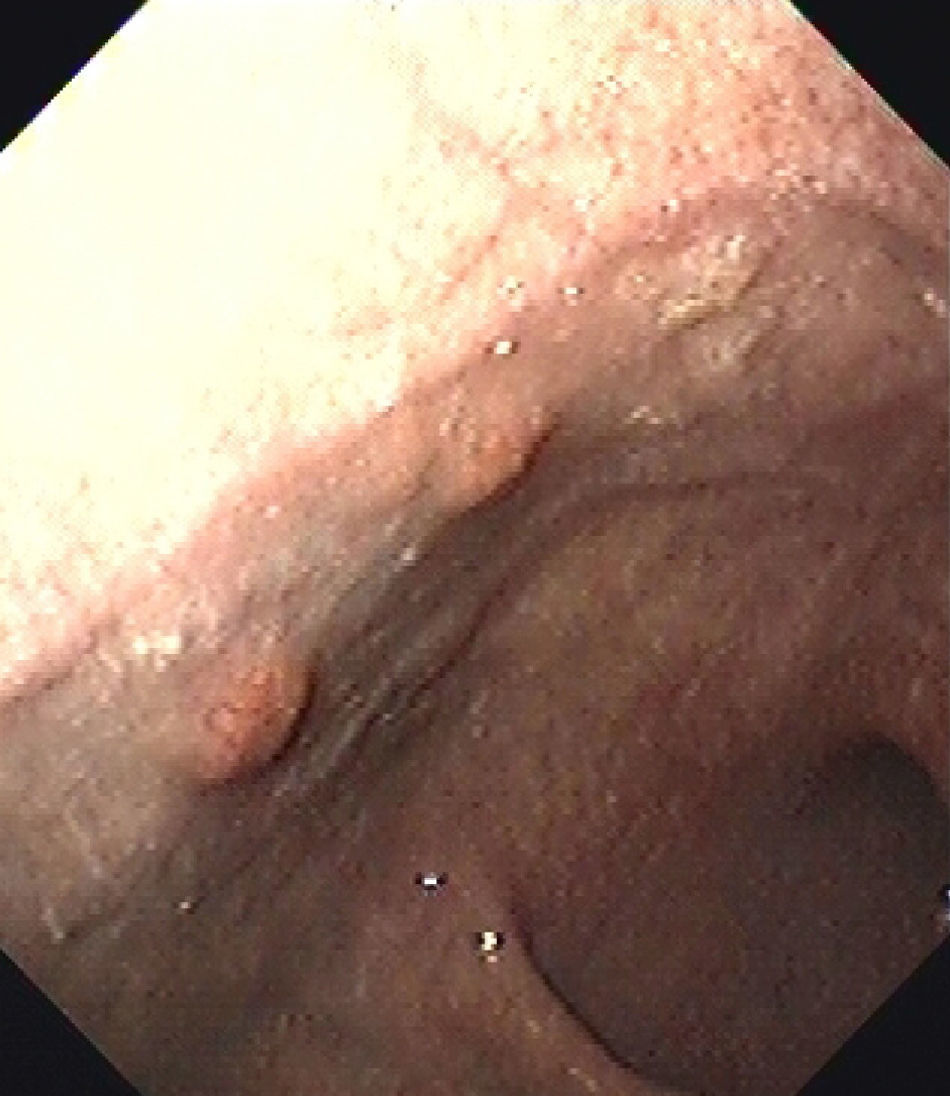
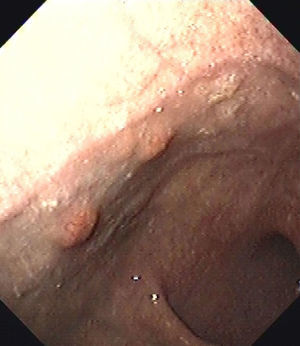
At the endoscopic control performed one year later, six new gastric polyps were seen (Fig. 1). Three of them were resected, and were found to be consistent with well differentiated gastric carcinoid tumors.
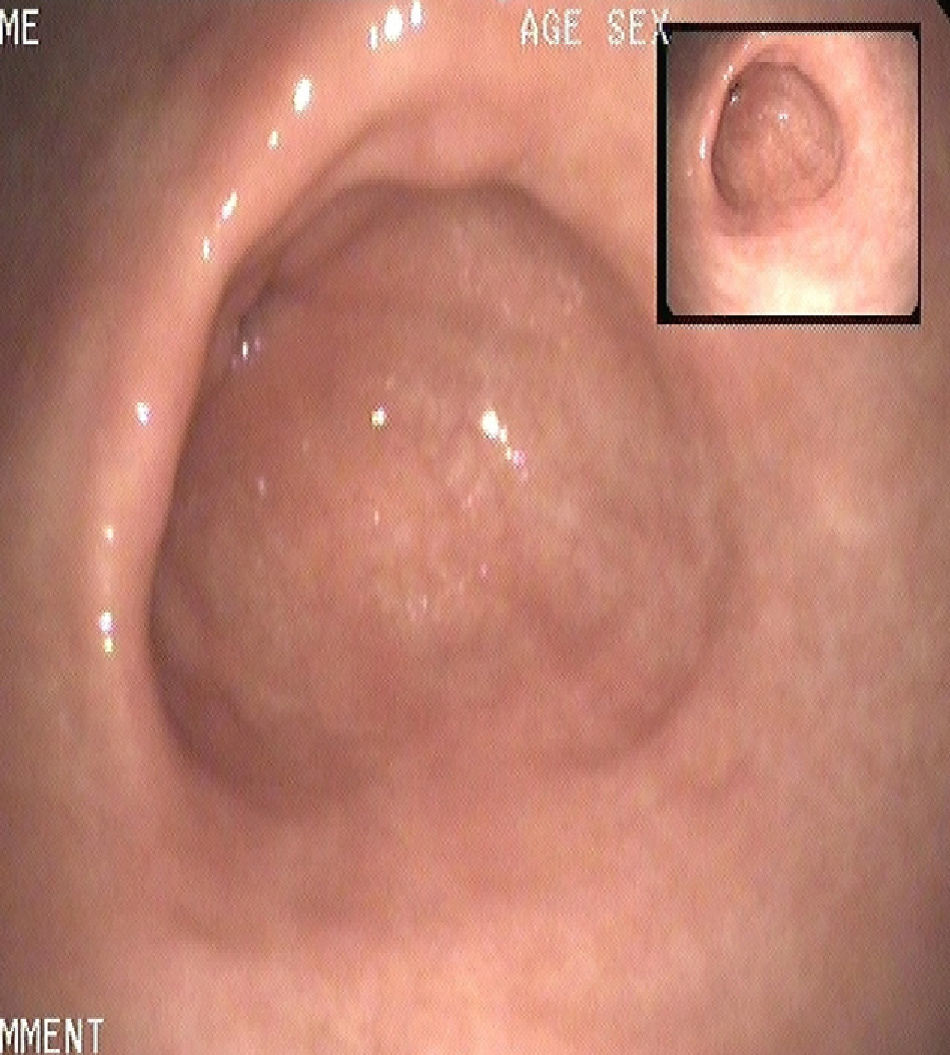
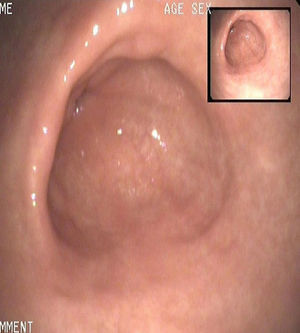
At that time, treatment for the relapse of the disease was started with lanreotide at monthly doses of 90mg for 12 months. Annual gastroscopies performed during the following three years showed an absence of tumors and disease remission (Fig. 2). One year after treatment, chromogranin A was within the normal range, 3nmol/L, and gastrin levels were slightly elevated to 167pg/mL.
Case 2A 36-year-old female patient with a personal history of autoimmune polyglandular syndrome type II with Addison's disease, primary hypothyroidism, and vitiligo. She was receiving hydrocortisone 20mg/day, fludrocortisone 0.05mg/day, and levothyroxine 100mcg/day. In a routine visit, the patient reported non-specific asthenia, and laboratory test results revealed anemia with a hemoglobin value of 10.5g/dL and an MCV of 99fL. She also had a vitamin B12 level of 45pg/mL (No.>200pg/mL) and a plasma gastrin level of 502pg/mL (No.<108pg/mL). A gastroscopy showed three polyps each less than one centimeter in size. The largest polyp (0.8cm) was resected, and a pathological examination revealed a well differentiated neuroendocrine tumor with Ki 67 < 2%. Treatment was administered with octreotide LAR 20mg monthly for 12 months, and in the next two annual endoscopic examinations the unresected polyps had disappeared, and no new neoplasms were found. Plasma gastrin level normalized after one year of treatment (101pg/mL).
Case 3A 28-year-old male with epigastric pain and intermittent nausea is reported. Laboratory tests found a plasma gastrin level of 1200pg/mL (No.<108pg/mL) and a chromogranin A level of 6nmol/L (No.<4nmol/L). Intragastric 24-h pH measurement showed a pH always higher than 4, and a gastroscopy revealed findings consistent with chronic atrophic gastritis and six polypoid lesions less than 0.8cm in size, which were all resected. Pathological examination disclosed well differentiated carcinoid tumors with a Ki 67<2%. At six months, a repeat gastroscopy revealed four new lesions less than one centimeter in size, which were resected and again showed well differentiated carcinoid tumors. Treatment was started with octreotide LAR 20mg/month by the IM route for six months. Gastroscopic monitoring at six and 12 months was normal, as well as plasma gastrin (103pg/mL) and chromogranin A (3nmol/L) levels.
Based on the courses of the three cases reported, treatment with somatostatin analogues appears to have an antiproliferative effect on well differentiated gastric carcinoids, and represents an alternative to other more invasive therapies. This is a well tolerated treatment with few side effects, but very expensive. Optimal treatment dosage and time, as well as its specific role in the guidelines for treatment of well differentiated gastric endocrine tumors, have not been elucidated yet. The reported cases show disease remission up to three years after treatment discontinuation.
Please, cite this article as: Cuesta Hernández M, et al. Tratamiento de carcinoides gástricos bien diferenciados con análogos de somatostatina. Endocrinol Nutr. 2013;60:37–47.