Most research in diabetes mellitus (DM) has been conducted in animals, and their replacement is currently a chimera. As compared to when they started to be used by modern science in the 17th century, a very high number of animal models of diabetes is now available, and they provide new insights into almost every aspect of diabetes. Approaches combining human, in vitro, and animal studies are probably the best strategy to improve our understanding of the underlying mechanisms of diabetes, and the choice of the best model to achieve such objective is crucial. Traditionally classified based on pathogenesis as spontaneous or induced models, each has its own advantages and disadvantages. The most common animal models of diabetes are described, and in addition to non-obese diabetic mice, biobreeding diabetes-prone (BB-DP) rats, streptozotocin-induced models, or high-fat diet-induced diabetic C57Bl/6J mice, new valuable models, such as dogs and cats with spontaneous diabetes, are described.
La mayoría de la investigación desarrollada en diabetes ha sido realizada mediante el uso de modelos animales, siendo su reemplazo todavía una quimera. Comparado con los primeros usos de estos modelos por la ciencia moderna, a partir del siglo XVII, el número de modelos animales disponible en la actualidad es muy elevado, ofreciendo nuevas perspectivas dentro de casi todos los aspectos de la enfermedad. Los abordajes que combinen estudios en humanos, in vitro y modelos animales son probablemente la mejor estrategia para mejorar el entendimiento de los mecanismos de la enfermedad aún subyacentes y, en este sentido, la elección del modelo que más se ajuste a dichos objetivos es determinante. Clasificados tradicionalmente en función de su patogénesis, en espontáneos o inducidos, cada modelo ofrece sus propias ventajas y desventajas. Se describen aquí los modelos de diabetes más comunes y, aparte del ratón Non-obese-Diabetic, la rata BioBreeding Diabetes-Prone, u otros modelos inducidos por estreptozotocina o dieta con alto contenido graso, se describen otros valiosos modelos de diabetes, como son el perro y el gato con diabetes espontáneas tipo 1 y tipo 2.
The consequences of diabetes mellitus (DM) on people's lives have motivated the search for better understanding of the mechanisms of the disease, as well as better treatments.1 Even though in vitro and in silico studies have improved in the last decades, they cannot completely replace the information inferred from animal models, given the complex etiology and multi-systemic interactions present in diabetes.2 Most of the research in diabetes is performed in animals3,4 and animal replacement5 is still a chimera. The combined approach of human, in vitro and animal studies is probably the best strategy to improve our understanding of underlying mechanisms.4
The modern, more standardised use of animal models of DM started during the 17th century by Brunner,6 though even earlier uses of animal research have been reported.7 Claude Bernard, father of ‘vivisection’, identified the existence of differentiated endocrine and exocrine pancreas using animal models.7 Some of the most important discoveries of biomedical research and DM were made with pancreatectomised dogs: the discoveries of pancreatic function by Von Mering and Minkowski8 and the insulin hormone by Banting and Best.9 Presently, a variety of animal models of DM are present and many additional advances have been possible: leptin's discovery, new insights into pathogenesis and complications and the development of new treatments, among others.2,10,11
These models are mainly classified based on which type of diabetes they mimic and whether they are spontaneous or induced.2,10–13 Furthermore, progress in genetics has allowed to generate specific transgenic models, almost à la carte, extending the range of spontaneous or more susceptible/resistant models.14 Sometimes, when they develop DM, the disease can be inconsistently classified as spontaneous or induced depending on the author,2,13 as they are genetically induced, but clinical signs and symptoms of DM appear spontaneously.
Another group of models that should be regarded is humanized models. They are developed either as spontaneous or induced diabetic models, and they have allowed research progress in regenerative medicine for diabetes, but also in many other fields (reviewed by Kennedy et al. 2016).15
Spontaneous diabetes modelsDiabetes occurs spontaneously in many animal species, including the horse, dolphin, and even hippopotamus, among many others (reviewed by E. Gale).3 Some of these species have provided important results as veterinary patients or as animal models, enabling better understanding of underlying mechanisms of DM. The principal advantages when compared with induced models are that they are presumed to share mechanisms of disease with the human condition, especially in polygenic models.3,11,12
The past decade has seen remarkable advances in the understanding of genetics and pathophysiology of spontaneous models of immune mediated diabetes and the creation of new models. The most commonly used spontaneous models of T1D are the non-obese diabetic (NOD) mouse and the BioBreeding Diabetes-Prone (BB-DP) rat.10 Other spontaneous T1D models include Long Evans Tokushima lean (LETL) rat and the New Zealand white rabbit. These models provide useful tools for the study of the autoimmune process and prevention of T1D. For T2D, the most frequently used spontaneous models are the Zucker fatty (ZFR) and Zucker diabetic fatty rats (ZDF) and the ob/ob and db/db mice.
Nevertheless, although their contribution is considerable, successful animal outcomes have failed to be translated to humans.4 Consequently, the choice of appropriate, single or combined animal models should be made to fit specific purposes, according to their validation to the aim.2,4 The most relevant, spontaneous models are described below.
The Non-Obese-Diabetic (NOD) mouse and the BioBreeding Diabetes-Prone (BB-DP) ratThe NOD mouse and the BB-PD rat are the most prominent and frequently used spontaneous models of T1D since they emerged 40 years ago.10,12
The first NOD mouse was established by intercrossing females derived from the JcI-ICR strain, a model for cataracts.16 NOD mice develop insulitis already at the age of 3–4 weeks, preceding subclinical β-cell destruction and insulinopenia. Final establishment of DM occurs between 12 and 30 weeks of age and animals can survive without insulin injections for weeks. Evident sexual dimorphism is present: 90% of females develop DM, whereas only 60% of males do.16
The BB-DP rat derived from Wistar strain intercrosses in the Bio-Breeding Laboratories.10 This rat shows classic clinical signs of insulinopaenia at 12 weeks or earlier. In contrast to NOD, insulin treatment is mandatory to assure survival of the BB rat, as ketoacidosis is severe and fatal.17
The pathogenic mechanisms leading to DM in both models are close to those described for human T1D.10 In the endocrine pancreas, autoimmune processes mediated by T cells, B cells, macrophages and natural killer cells lead to insulitis and islet loss.16,17
Autoantibodies have also been identified in both models, but, as in humans, their pathogenic role has not been confirmed. Genetic studies have confirmed the role of the MHC region, both in NOD and BB-PD.16,18 The role of virus infections, dietary factors, vitamin D or immunosupressors like cyclosporine-A in the pathogenesis of DM, have been widely studied in both BB and NOD, also for the development of new therapies.
Zucker fatty rat (ZFR) and Zucker diabetic fatty rat (ZDF)The ZFR was first identified by Zucker in 1961, in the rat stock of Sherman and Merck, USA.11 Also named as (fa/fa) fatty or obese rat (Leprfa), its phenotype is derived from an autosomal recessive mutation (fa) in a gene on chromosome 5, which leads to dysfunction in leptin receptor signaling in the hypothalamus.11 Following this mutation, hyperphagia and obesity develop already at four weeks of age. This phenotype leads to mild hyperglycaemia and insulin resistance (IR), glucose intolerance, hyperlipidaemia, hyperinsulinaemia and hypertension. The principal causes of the mild glucose intolerance are hepatic metabolic defects.11 ZFR have mostly been used to study insulin sensitisers and antiobesity agents.
Derived from the ZFR, the ZDF was selectively inbred for hyperglycaemia. Both ZFR and ZDF are models of the metabolic syndrome and T2D. ZDF is less obese and more insulin resistant and develops mild diabetes.19 There is marked sexual dimorphism and males are more susceptible to develop diabetes, normally from 7 to 10 weeks of age. On the contrary, females show a phenotype, which is closer to the ZFR. They are normally used as non-diabetic controls for their male littermates.11
Unlike the ZFR, they do not show compensatory insulin over-secretion in response to peripheral IR and β cells are ultimately damaged, following apoptosis, due to the higher secretion demand, even though proliferative mechanisms are not affected.20 Marked lipotoxicity and down-regulation of GLUT-2 and GLUT-4 glucose transporters have also been demonstrated.11 ZDF have been used for the study of mechanisms of IR and β-cell dysfunction.
The ob/ob, db/db, Lepob, Leprdb mouseThe ob/ob or Lepob mouse (obese mouse), with C57BL/6J strain background, has a monogenic, autosomal, recessive mutation (obese) on chromosome 6. The mutation in ob/ob mice has been pinpointed to the leptin gene. Leptin, which is absent in this homozygously ‘obese’ mouse, is mainly synthesised in adipose tissue and causes appetite suppression and energy expenditure, and modulates IR.21 The lack of leptin leads to hyperphagia, decreased energy expenditure and obesity, high levels of neuropeptide Y (an orexigenic peptide) and hypercorticosteronism, which also contributes to IR.11 Mice gain weight early and rapidly and end up weighing three times wildtype controls. The diabetes-like syndrome of the ob/ob mouse is characterised by hyperglycaemia, mildly impaired glucose tolerance, severe hyperinsulinaemia, sub fertility and impaired wound healing.22 Histological examination of the pancreas shows hypertrophy and hyperplasia of pancreatic islets.23 IR is caused by reduced insulin binding to its receptors, impaired insulin receptor autophosphorylation, and reduced signal transduction.11 This model has been used to test body weight loss and antiobesity treatments, insulin sensitisers and antihyperglycaemic agents. The db/db or Leprdb mouse shares the same characteristics of ob/ob, but its mutation is sited in the leptin receptor gene.11
The diabetic catDiabetes is one of the most common spontaneous endocrine diseases in cats, and it is estimated that approximately 80% of naturally occurring diabetes is similar to human T2D, with important IR as the principal cause,24 although the reasons for this IR are not fully understood.24
The most common clinical signs of feline diabetes mellitus are polyuria, polydipsia, diminished activity, weight loss and polyphagia.25 Weakness, vomits and diarrhea can be also present, and in lower proportion of cases (3–8%) ataxia and plantigradism both related to diabetic neuropathy.25
Genetics play a role in the pathogenesis of fDM in cats, as reflected by the increased risk associated with certain breeds (e.g. Burmese and Russian blue),26–28 but environmental factors are also important.24 Indeed, the major risk factor for the development of DM in this model is obesity, together with physical inactivity.27,29 In fact, obese cats are approximately 4 times more likely to develop diabetes compared with those with optimal body weight.24 Moreover, weight gain in cats is also associated with IR, which is worse in males and older animals.27,29,30 However, there are no available reports on the effect of sex steroids on weight and IR.24 In early stages of feline diabetes, IR can be reversed by weight loss and diabetes remission has been reported (reviewed by Gostelow et al.).31
Some similarities to human obesity and T2D are observed, comparing findings from obese and lean cats. Indeed, the expression of insulin signaling genes (IRS-1, IRS-2, PI3-K…) in liver and skeletal muscles is lower in obese cats.24 Adipose tissue-derived hormones’ mRNA expression, secretion and action are affected, as is the case of adiponectin (anti-inflammatory and insulin sensitising hormone), which decreases with obesity and DM.30,32 Cats with diabetes show higher blood leptin concentrations,32 although no differences have been found in mRNA expression when compared to lean cats.32
Obesity is also considered a chronic inflammatory process, where adipose tissue secretes pro-inflammatory cytokines, such as TNFa, which is increased in the fat of obese cats.24
However, not all obese cats become diabetic. In this sense, the most suitable explanation is probably the existence of secretory dysfunction of the β-cell, but available data are insuficient to draw conclusions, for the time being.24 Amyloid deposition, glucotoxicity and lipotoxicity have all been proposed as possible mechanisms.24 Glucotoxicity has been shown to damage β-cell function and survival in cats.33 In a recent study, after ten days of prolonged, induced hyperglycaemia in healthy cats, 50% of their β-cell mass was lost. Apoptosis and systemic inflammatory responses were detected.24 Similar mechanisms have been described in humans.34 The role of lipotoxicity in feline diabetes, as in humans, seems to enhance the effects of glucotoxicity.24
The most accepted hypothesis to explain the loss of β-cell function in cats is their destruction by amyloid deposition. Obese cats with IR, as humans and other primates show amyloid deposits, which can, however, also be found in healthy cats.35,36 On the other hand, these deposits are absent in other species like dogs and rodents.36
The diabetic dogIn 1921, Marjorie, an induced diabetic crossbreed dog, received insulin therapy for the first time, paving the way for treatment of human patients.9
Although the first report of spontaneously diabetic dogs was published in 1951,37 canine DM (cDM) was only very recently proposed as a spontaneous animal model of human autoimmune diabetes.38 This is supported by clinical presentation, the existence of both purebred and outbred animals, shared environment and common pathogenesis, with the presence of auto-antibodies and genetic risk.38,39 Our shared environment represents an important advantage, when compared to other animal models of DM and special interest in comparative research can be undertaken to investigate the interaction between genetic and environmental factors. Despite the importance of rodent models, dogs are genetically closer to humans.
Diabetic dogs show classical symptoms of T1D: polydipsia, polyuria and weight loss, associated with hyperglycaemia and glucosuria.24 Diagnosis is mostly based on these and other clinical signs, like cataracts (10–20% cases), coexisting with hyperglycaemia or increased fructosamine and glucosuria. Clinical history, physical examination and diagnostic tests are crucial to help differentiate the type of cDM, needed to provide the appropriate treatment and to determine prognosis. Treatment includes insulin injections in most of the cases, administered once or twice daily, but also exercise, dietary modifications and sterilisation of intact females.24
Types of cDM are established based on etiology,24,38,39 following the most accepted classification, adapted from human classification.38 Although certain differences among populations exist,40,41 the most common form is insulin-deficient diabetes (IDD), which is similar to human T1D. Other common types of cDM are dioestrus and secondary DM (to pancreatitis or hyperadrenocorticism).
The role of immunity in cDM is supported by the presence of islet cell-(ICA), GAD65-, IA2- and proinsulin antibodies,39,42,43 although a recent report questions this role.44 An association between cDM and the MHC class II genes, the Dog Leukocyte Antigen (DLA), has also been demonstrated and protective and risk haplotypes have been identified.45DLA haplotype distribution in different dog breeds has been compared to that found for HLA in different human ethnic groups.39,45 Age of onset ranges from 5 to 12 years in most studies,40 although rare, juvenile forms of cDM also exist.25 Autoimmune processes, clinical presentation and middle-age onset, suggest that cDM could be a model for human latent autoimmune diabetes of the adult (LADA).39,46
Prevalence of cDM ranges between 0.0005 and 1.5%, depending on both geographical regions and breeds,38,40,41,47,48 suggesting gene-environment interactions. However, most epidemiological studies have been performed in northern European and North American populations,40,41,47,48 with specific cultural and demographic characteristics. Attending to these reports, the most frequently affected breeds are Australian terrier, Keeshond, Schnauzer, Miniature Poodle, Samoyed and Cairn Terrier.38,40,47 Other breeds, like Boxer and German shepherd, seem to be protected against the development of cDM.39
Glucose control in small diabetic animalsManagement of DM in small animals (dog and cat) requires a broad clinical approach, including symptom assessment and laboratory and point-of-care (POC) tests. Total blood or plasma glucose concentrations (cat: >250mg/dL; dog: >200mg/dL) and glucosuria confirm the diagnosis in animals with the classical clinical signs.24
The treatment of cDM is mainly based on porcine insulin, administered subcutaneously, with some other strategies depending on the type of cDM, such as oophorectomy and histerectomy for entire female dogs. Once clinical signs are evident and permanent, insulin is needed.25 In cats, the treatment is similar to cDM, but in initial or pre-diabetic stages other strategies can be applied: oral agents (i.e. glipizide), body weight loss due to the increase of physical activity or specific feed regime and formulated diets, among others.49
Initial insulin regime usually starts with 0.3–0.5UI/kg in dogs and 0.25–0.5UI/kg in cats, and is adjusted thereafter, according to individual response, assessed by serial glucose measurements.25,49 The most frequently used methods, both for owners and practitioners, are urine test strips25 and blood glucose determinations performed by specialised laboratories or by domestic, or POC, portable blood glucose meters (PBGM). Continuous blood glucose monitoring has also been tested,49 but its clinical use is still exceptional.
For long-term evaluation of glucose control, glycated protein assays are also available. Fructosamine, the most frequently used measurement, reflects glucose control for the previous 2–3 weeks and may allow distiction of DM from stress hyperglycaemia. It is considered a standard diagnostic and monitoring tool for cDM and for fDM in not recent-onset or mild cases (cat: >400μmol/L; dog: >350μmol/L).25,49
Self-monitoring with PBGMs in humans is pivotal in the management of insulin-treated diabetes, where frequent glucose measurements and insulin dose adjustment have proved to reduce disease complications.50 In veterinary medicine, adequate glycaemic control increases survival in both cDM and fDM. In this context, PBGM allow owners and practitioners to obtain glucose determinations easily and make immediate therapeutic decisions.
Specific PBGMs have been developed for small animals,51 which offer reliable glucose measurements and might be the best option for them, but are not widely available. Thus, those initially developed for humans are commonly used in both cDM and fDM, and other animals, too, although there has been some concern regarding their reliability.51
Induced diabetes modelsInduced animal models are probably the most frequently used type of DM models, since they are easier to generate than the spontaneous models. They fit adequately to many research purposes, which are less focussed on the autoimmune process and more on hyperglycaemia itself, obesity or the metabolic syndrome.
Two major ways of developing diabetes are described for induced models. Surgical models consist of partial or complete ablation of the pancreas, whereas non-surgical models can be generated by the administration of toxic substances with β-cell trophism (i.e. alloxan or streptozotocin (STZ), hyper-caloric diets (e.g. high-fat, or high-sucrose), immunosuppresors or even by viral infections (e.g. Coxsackie B virus).10,52,53 Furthermore, both surgical and non-surgical methods can be combined to mimic special or more complex types of DM (e.g. combining high-fat diet with STZ, or under specific physiological conditions, like in pregnancy).54
Surgically induced modelsThe most common way of inducing diabetes surgically is pancreatectomy, although other methods are available.11 Total pancreatectomy, which led to the famous discovery of insulin in dogs8 and was later translated to rodents for the generation of T1D or T2D-like diseases, is less frequently used nowadays, except in specific areas, such as islet transplantation or β-cell regeneration.55 Partial pancreatectomy has been performed in many species (dog, pig, rabbit, rat, mouse),54 including large animals. After the removal of 50–95% of their pancreatic mass,54,55 animals develop a mild diabetic state, with hyperglycaemia and IR, which can be combined with many other physiological conditions (obesity, pregnancy, etc.). In fact, it has been used to study intrauterine developmental disorders in gestational diabetes. The development of mild, insulin-independent hyperglycaemia (from 150 to 200mg/dl) is the main advantage of this technique. However, it is invasive and requires a high degree of expertise, since up to 20% post-surgical mortality has been described, and severe hypoglycaemia and pancreatic exocrine insufficiency are also common.54
Chemically, toxic or drug induced modelsSeveral drugs and toxics with affinity for the β-cell10,53 have been used to develop models of DM. The most commonly chosen are STZ and alloxan, although others, like vacor or dithizone, have also been used.10
Administered at different doses, intervals or routes (i.e. intraperitonial vs intravenous), both STZ and alloxan may lead to disorders mimicking T1D, T2D, pre-DM or other conditions.54 If high doses are applied, the model mimics human T1D by irreversible loss of β-cells. If lower doses are given, mild impairment of insulin secretion, a condition resembling T2D, is obtained.53
A single, large dose (i.e. 200mg/kg for mice) of STZ leads to fulminant diabetes by direct toxic effects, but of different severity depending on the route of administration and animal susceptibility.53,56 On the other hand, the same drug, administered in multiple, small doses (i.e. 40mg/kg for mice) for several days (1–5 daily applications), causes a process that resembles human T1D, with immune destruction and insulinopaenia.56 This application has shed light on the immunological pathways of insulitis and β cell death.57 However, unlike spontaneous DM, the disease develops even without the intervention of T and B lymphocytes.56
Alloxan is a synthetic pyrimidine derivative first synthesised in the XIX century,58 that causes necrosis, via a selective, toxic effect on β-cells, like STZ.59 Its use was first reported in rabbits (1943),11 where STZ is inefficacious. On the other hand, Guinea pigs seem to be resistant to alloxan (reviewed by Srinivasan et al.).11
Diet induced modelsThe ongoing epidemics of obesity, metabolic syndrome and T2D are mainly attributed to over-nutrition and reduced physical activity. In animals, dietary manipulation is commonly employed to study this human situation. Many species are prone to develop diet-induced IR and glucose-impairment: rodents, cats, squirrels, pig, apes… but rodents are the most extended for research purposes.60 Since the first experiments with high-fat diets (70% content) were performed in rats in the 1940s,61 diet-induced models have become one of the most widespread models for the study of T2D.62
Rodent diets are formulated attending to their daily caloric needs (10–15kcal/day) and are provided ad libitum. Standard “chow diet” usually consists of 65–70% carbohydrate, 20–25% vegetable proteins and 5–12% fat, for an approximate caloric intake of 2900kcal/kg. Increasing the proportion of fat (up to 85% of total caloric content) or simple carbohydrates (30–60% caloric intake of i.e. sucrose), or adding high salt or cholesterol to the diet, provides a variety of animal models to study obesity, IR, hypertriglyceridaemia, hypertension, atherosclerosis and other related disorders.
The most established rodent model in this context is the C57BL/6J mouse, given a hypercaloric, high-fat (4730kcal/kg, 40% fat) or very high fat (5240kcal/kg, 60% fat) diet. Variable, strain-dependent response to dietary intervention has been reported.52,63
Additionally, dietary intervention on genetically manipulated animals gives a wide range of models with known genetic roles, or can be combined with chemical induction of DM.62
The High-Fat Diet Induced Diabetic C57Bl/6J mouse (HFDID)Marked obesity, IR, hyperinsulinaemia, hyperglycaemia and glucose intolerance are the main features of HFDID C57Bl/6J mice.52 They also manifest peripheral leptin resistance [108], and a prolonged hyperglycaemic response to stress.52
Following the most common protocol with young animals (4–6 weeks of age), the development of hyperglycaemia occurs over long periods of time, since β-cells maintain high insulinaemia to compensate for IR.64 At least 10–12 weeks are necessary to obtain an evidently obese and diabetic phenotype, and although the strain is considered to be obesity-prone, individual variability exists.60
The main advantage of this animal model is that it reflects the interaction between complex genetic and environmental risk factors, compared to the Lepob/ob mouse (C57Bl/6J background), which is extremely genetically determined.11 In this sense, the HFDID C57Bl/6J mouse has been used in the study of impaired glucose tolerance, T2D and its complications as well as for preclinical drug testing.
Evaluation of glucose metabolism in the C57Bl/6J mouseCharacterisation of glucose metabolism in mice is mainly performed using FPG, glucose tolerance tests (GTT) and the insulin tolerance test (ITT), but model assessments and clamps can be applied, too. Although all the procedures are extensively applied in rodent phenotyping, there is a lack of standardisation among protocols, glucose/insulin doses, analytical devices employed, etc.62,65,66 No single test is suitable under all circumstances and each one should be applied following uniform criteria.66
Initial screening is based on single blood glucose measurements, usually performed with a “human” PBGM. FPG concentrations represent an estimation of IR, especially if simultaneous insulinaemia is measured. Both parameters are used to calculate the homeostatic model assessment (HOMA) indexes, that estimate IR (HOMA-IR) or β-cell function (HOMA-%B).67 In humans, this equation shows strong linear correlation with the euglycaemic, hyperinsulinaemic clamp, the gold-standard in the assessment of glucose metabolism,67 but in rodents it has not been validated, although it is used for group comparison.
Glucose tolerance tests (GTT) consist of the administration of a standardised glucose load and the measurement of glycaemic response thereafter. Performed orally (OGTT), intravenously (IVGTT) or intraperitoneally (IPGTT), the route of administration can be chosen to appraise certain aspects, such as gut absorption or incretin activity.
The reviewed literature,62 demonstrates a lack of consistency among the different protocols for GTT, and errors that affect rodents’ normal physiology and interfere with the results are common.62 One of the most frequent is an excessively long fasting period (18h, overnight), which leads to weight loss and hepatic glycogen depletion.68 The fact that mice are nocturnal eaters intensifies this catabolic state.69 In this sense, the most carefully standardised protocol for GTT in mice was described in 2008 by Andrikopoulos et al.,65 establishing that in conscious or anaesthetised animals, after a fasting period of 6h (from 8:00 to 14:00), the most homogeneous results between groups are obtained following an oral glucose load of 2g/kg.65 The area under the curve (AUC), peak, and baseline glucose values are the most established glucose variables in the GTT.
The insulin tolerance test (ITT), also known as insulin sensitivity test, consists of the evaluation of glucose response to a known insulin load, intraperitoneally (IPITT) or intravenously (IVITT), after a fasting period. Baseline glucose, glucose decline, AUC and nadir (lowest glucose value) are the most frequently used variables in this case, to obtain an estimation of IR.66
The hyperinsulinaemic-euglycaemic clamp is the gold standard for the evaluation of IR. Its name derived from the “clamping” on glucose at a desired fixed concentration (e.g. 100mg/dL). Glucose infusion rate (GIR), the glucose amount necessary to maintain euglycaemia in the presence of a constant insulin infusion, is the main outcome of the test, and is inversely related to IR.
In addition to these procedures, long-term evaluation of glucose metabolism is also performed in mice by means of HbA1c,70 which, in humans, also serves as a diagnostic test for DM (Table 1). Different methods have been used to measure HbA1c in mice: from ion-exchange high-performance liquid chromatography (HPLC) to antibody based approaches.70,71 However, a lack of consistency among studies is present and there is no cut-off point defined for DM diagnosis. From the point of view of animal welfare,5 standardisation of a test such as HbA1c would be very relevant: POC analysers could provide reliable results with small, single blood samples (1μL).70 Hence, for correct glucose monitoring in mice, standardisation of all of these procedures and analyses is desirable, since their variability makes comparisons between studies difficult. This fact complicates the per se demanding inter-species extrapolation.62
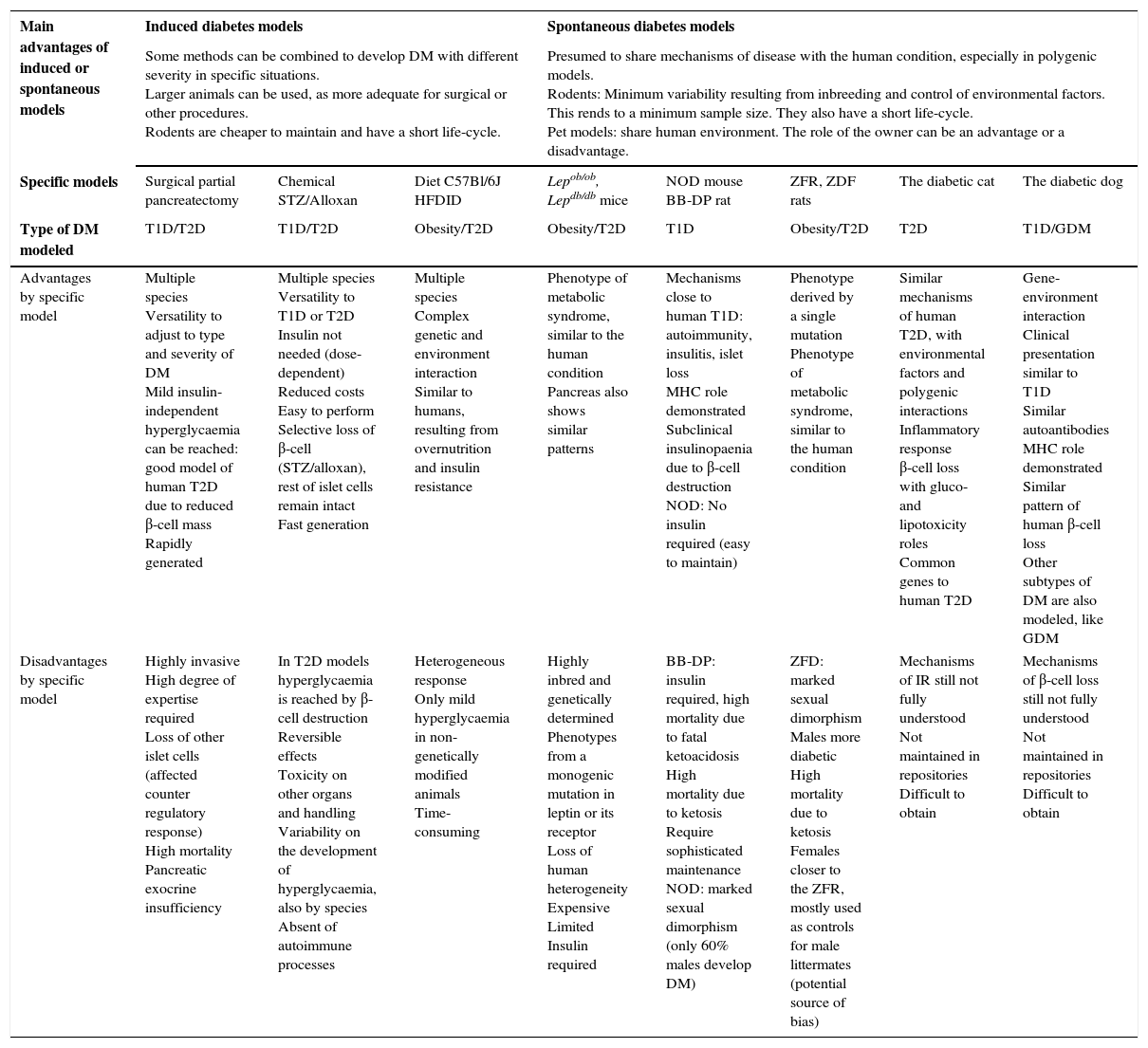
Advantages by group, and advantages and disadvantages of each described animal model.
| Main advantages of induced or spontaneous models | Induced diabetes models | Spontaneous diabetes models | ||||||
|---|---|---|---|---|---|---|---|---|
| Some methods can be combined to develop DM with different severity in specific situations. Larger animals can be used, as more adequate for surgical or other procedures. Rodents are cheaper to maintain and have a short life-cycle. | Presumed to share mechanisms of disease with the human condition, especially in polygenic models. Rodents: Minimum variability resulting from inbreeding and control of environmental factors. This rends to a minimum sample size. They also have a short life-cycle. Pet models: share human environment. The role of the owner can be an advantage or a disadvantage. | |||||||
| Specific models | Surgical partial pancreatectomy | Chemical STZ/Alloxan | Diet C57Bl/6J HFDID | Lepob/ob, Lepdb/db mice | NOD mouse BB-DP rat | ZFR, ZDF rats | The diabetic cat | The diabetic dog |
| Type of DM modeled | T1D/T2D | T1D/T2D | Obesity/T2D | Obesity/T2D | T1D | Obesity/T2D | T2D | T1D/GDM |
| Advantages by specific model | Multiple species Versatility to adjust to type and severity of DM Mild insulin-independent hyperglycaemia can be reached: good model of human T2D due to reduced β-cell mass Rapidly generated | Multiple species Versatility to T1D or T2D Insulin not needed (dose-dependent) Reduced costs Easy to perform Selective loss of β-cell (STZ/alloxan), rest of islet cells remain intact Fast generation | Multiple species Complex genetic and environment interaction Similar to humans, resulting from overnutrition and insulin resistance | Phenotype of metabolic syndrome, similar to the human condition Pancreas also shows similar patterns | Mechanisms close to human T1D: autoimmunity, insulitis, islet loss MHC role demonstrated Subclinical insulinopaenia due to β-cell destruction NOD: No insulin required (easy to maintain) | Phenotype derived by a single mutation Phenotype of metabolic syndrome, similar to the human condition | Similar mechanisms of human T2D, with environmental factors and polygenic interactions Inflammatory response β-cell loss with gluco- and lipotoxicity roles Common genes to human T2D | Gene-environment interaction Clinical presentation similar to T1D Similar autoantibodies MHC role demonstrated Similar pattern of human β-cell loss Other subtypes of DM are also modeled, like GDM |
| Disadvantages by specific model | Highly invasive High degree of expertise required Loss of other islet cells (affected counter regulatory response) High mortality Pancreatic exocrine insufficiency | In T2D models hyperglycaemia is reached by β-cell destruction Reversible effects Toxicity on other organs and handling Variability on the development of hyperglycaemia, also by species Absent of autoimmune processes | Heterogeneous response Only mild hyperglycaemia in non-genetically modified animals Time-consuming | Highly inbred and genetically determined Phenotypes from a monogenic mutation in leptin or its receptor Loss of human heterogeneity Expensive Limited Insulin required | BB-DP: insulin required, high mortality due to fatal ketoacidosis High mortality due to ketosis Require sophisticated maintenance NOD: marked sexual dimorphism (only 60% males develop DM) | ZFD: marked sexual dimorphism Males more diabetic High mortality due to ketosis Females closer to the ZFR, mostly used as controls for male littermates (potential source of bias) | Mechanisms of IR still not fully understood Not maintained in repositories Difficult to obtain | Mechanisms of β-cell loss still not fully understood Not maintained in repositories Difficult to obtain |
In conclusion, until in vitro and in silico studies could replace them, the use of animals diabetes research is still mandatory. The correct election of one, or even various, animal models that best fit for a specific research purpose, could improve the translational value of the results and presumably reduce ulterior animal studies. Lack of fit of previous models, or failures in the translation of the results, are two reasons why new animal models like both dog and cat are being considered, regardless of specific transgenic models here not described. Combined approaches in vitro, in silico, clinical studies with animal models are still today the best strategy to explore new insights in DM.
Conflict of ineterstThe authors declare no conflict of interest.