To assess the incidence of 131I-induced sialadenitis (SD) in patients with differentiated thyroid cancer (DTC), to analyze clinical and other factors related to metabolic radiotherapy that may predict the lack of response to conventional medical therapy (CMT), and to determine the effectiveness of intraductal steroid instillation in patients failing CMT.
Material and methodsFifty-two patients with DTC, 45 females (86.5%) and 7 males (13.5%) with a mean age of 44.21±13.3 years (r=17–74) who received ablation therapy with 131I after total thyroidectomy. Patients with diseases and/or medication causing xerostomia were excluded. Patients underwent salivary gland scintigraphy with 99Tc (10mCi).
ResultsEighteen patients (34.62%) had SD and received antibiotics, antispasmodics, and oral steroids for 15 days. They were divided into two groups: responders to medical therapy (n=12, age 44.3±14.4 years, 2 men [17%], 10 women [83%], cumulative dose 225±167.1mCi) and non-responders to medical treatment, who underwent steroid instillation into the Stensen's duct (n=6 [33%], 2 men [33%], 4 women [67%], age 50±13.8 years, cumulative dose 138.3±61.7mCi). Scintigraphy showed damage to the parotid and submaxillary glands.
ConclusionIncidence of 131I-induced sialadenitis was similar to that reported by other authors. Age, mean cumulative dose of 131I, and involvement of parotid and submaxillary glands did not condition response to CMT; however, male sex was a conditioning factor. Symptom persistence for more than 15 days makes instillation into the Stensen's duct advisable. This is an effective and safe method to avoid surgical excision of salivary glands.
Estudiar la incidencia de sialoadenitis (SD) por 131I en pacientes con cáncer diferenciado de tiroides (CDT), analizar los factores clínicos y otros vinculados a radioterapia metabólica que puedan predecir la falta de respuesta al tratamiento médico convencional (TMC) y determinar la eficacia de la instilación intraductal del Stenon (ITS) en pacientes con fracaso al TMC.
Material y métodosCincuenta y dos pacientes con CDT, 45 mujeres (86,5%) y 7 hombres (13,5%), con edad media 44,21±13,3 años, que postiroidectomía total recibieron dosis ablativa de 131I. Excluimos individuos con enfermedades/medicación causantes de xerostomía. Realizamos gammagrafía de glándulas salivales con 99Tc (10mCi).
ResultadosPresentaron SD 18 pacientes (34,62%) tratados con antibióticos, antiespasmódicos y corticoides vía oral durante 15 días. Se les dividió en 2 grupos: respuesta al tratamiento médico n=12 (67%), edad 44,3±14,4 años, 2 hombres (17%), 10 mujeres (83%), dosis acumulativa 225±167mCi; y sin respuesta al tratamiento médico, n=6 (33%), a los que se instiló corticoides en conducto de Stenon; edad 50±13,8 años, 2 hombres (33%), 4 mujeres (67%), dosis acumulativa 138,3±61,7mCi. Demostramos lesiones en la gammagrafía de glándulas parótidas y submaxilares.
ConclusiónLa incidencia de sialoadenitis por 131I fue similar a la descripta por otros autores. La edad, la dosis media acumulada de 131I y la afectación de glándulas parótidas o submaxilares no condicionaron diferente respuesta al TMC, solo relacionada con el sexo masculino. La persistencia de síntomas durante más de 15 días hace recomendable la ITS de corticoides, método eficaz y seguro para preservar dichas glándulas ante otras opciones como la exéresis quirúrgica.
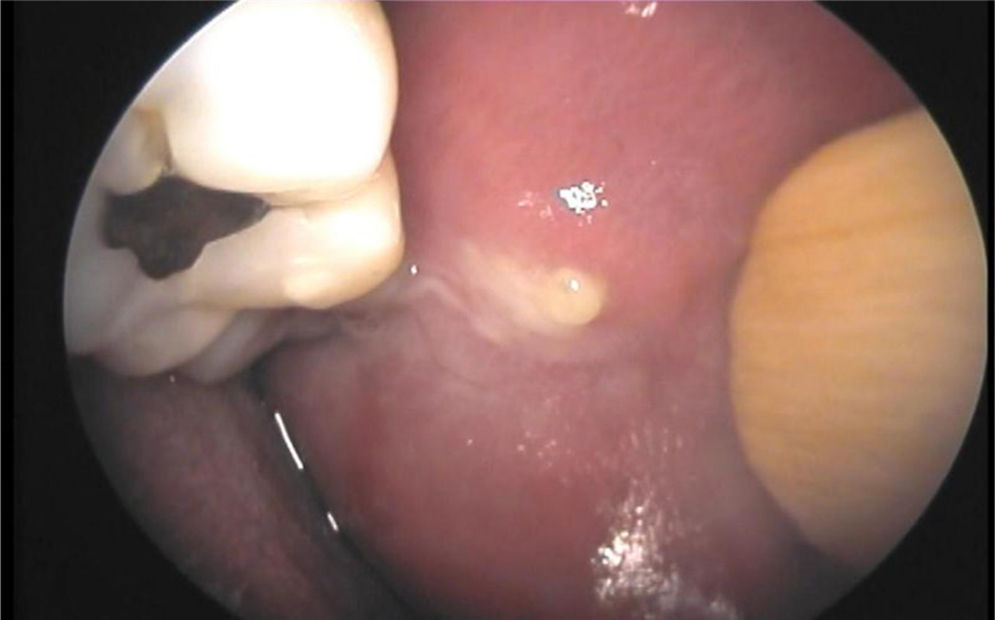
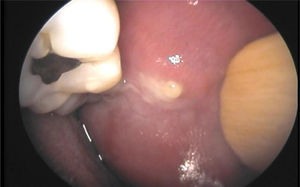
Ablation therapy with radioiodine (131I) for differentiated thyroid cancer (DTC) has a known effect on salivary glands called sialoadenitis.1 Clinical signs of sialoadenitis include pain, swelling, decreased salivation and, in some patients, purulent secretion (Fig. 1). Xerophthalmia and nasolacrimal obstruction have also been reported as complications of ablation therapy.2 Persistence of symptoms of salivary and lacrimal dysfunction may compromise quality of life and cause complications in the affected patients.3,4
Salivary gland swelling related to radiation would apparently depend on the dose administered; parotid glands, because of their anatomical structure, are much more sensitive to radiation than submaxillary glands.5 Cells from the salivary gland and ductal parenchyma contain a sodium/iodine transporter that also confers them a greater capacity to concentrate the radionuclide.6 Sialoadenitis may occur early, within 48h of treatment, or up to 3–6 months after therapy administration.
Some reviews have reported an incidence of acute sialoadenitis after administration of 131I ranging from 24% to 67%, and an incidence of chronic sialoadenitis ranging from 11% to 43%;7–9 salivary gland damage is shown by scintigraphy in 10–60% of patients with acute or chronic symptoms.5,10,11
Prophylactic measures to promote clearance of intraglandular radioiodine and prevent this complication have included intake of large amounts of fluid, lemon juice, or sour candy. Silberstein et al. used in a group of patients pilocarpine as a secretagogue for salivary glands, but the drug was not successful for preventing sialoadenitis in their study.12 An additional measure to decrease the effect of 131I in salivary glands is the use of recombinant human thyroid-stimulating hormone (rhTSH) before administration of the ablative dose. This has been shown to decrease radiation damage in tissue, but additional studies comparing the use of rhTSH and thyroxine removal are needed.13,14
Chronic obstructive changes in ductal epithelium are seen in patients with chronic juvenile recurrent parotiditis,15 and also after radioactive iodine therapy, radiation therapy to the neck, and in autoimmune diseases with salivary gland involvement such as Sjögren's syndrome.4 These conditions are usually not adequately diagnosed by ultrasonography, because gland parenchyma often shows non-specific hypoechogenic changes. Sialoendoscopy may help diagnose ductal changes. When sialoadenitis exists, ductal epithelium has a whitish, thickened, and rigid appearance, and the intraductal circular lines usually seen in normal ducts are not visualized. Fibrinous exudates, and a trend to ductal stenosis in some cases, may also exist.16
Obstructive changes are treated by corticosteroid instillation and, if required, removal of mucus plugs under endoscopic visualization or by gland massage or squeezing. This procedure may prevent, or at least delay, persistent ductal inflammation and gland changes leading to stenosis; endoscopic dilation of stenoses may also be used.17–21
Corticosteroids are usually instilled for 6 weeks, and this is the regimen used in our study.
This study examined the incidence of sialoadenitis induced by 131I in a patient population with DTC, analyzed which clinical factors and/or factors related to metabolic radiotherapy could be predictors of lack of response to standard medical treatment (SMT) for sialoadenitis, and assessed the efficacy of intraductal instillation of corticosteroids in patients with SMT failure.
Patients and methodsFifty-two patients with DTC, 45 females (86.5%) and 7 males (13.5%) with a mean age of 44.21±13.3 years (range 17–74), who received ablative doses of 131I after total thyroidectomy were included in the study. Sialoadenitis was defined as pain and inflammation in the salivary gland region. Subjects diagnosed with diabetes mellitus, Sjögren's syndrome, or smoking, and those taking drugs causing xerostomia were excluded.
All patients underwent salivary gland scintigraphy after IV injection of technetium-99 (99Tc), 10mCi (370MBq) for a weight of 70kg, under fasting conditions and 30min after an acid stimulus (lemon juice), assessing radionuclide concentration and elimination. Development of sialoadenitis was assessed in all patients, and those with the condition were divided into groups with response to medical treatment (RMT) and with no response to medical treatment (NRMT), treated by instillation of corticosteroids into Stensen duct.
Patients with sialoadenitis were initially treated with antibiotics (amoxicillin–clavulanate, levofloxacin), antispasmodics, and oral corticosteroids for 15 days; this was considered as the SMT. Intraductal instillation of corticosteroids may be combined with antibiotics if saliva with purulent contents and gland inflammation are detected. Instillation is performed on an outpatient basis under local anesthesia. A 4% lidocaine spray is applied to the oral mucosa, and papilla of the excretory duct is dilated using a conical tip dilator. The Stensen duct is canalized using the soft external sheath of a Butterfly no.° 22 needle (0.9mm) adapted to a syringe, and the desired drug is instilled.
Criteria for selecting patients with salivary gland symptoms treated with 131I who received intraductal instillation included:
- 1.
Permanent or intermittent gland inflammation and/or swelling.
- 2.
Pain in the salivary gland area, either spontaneous or during meals.
- 3.
Bitter or altered taste of saliva.
- 4.
Suppuration through the excretory duct.
- 5.
Pain or discomfort in the infraauricular or submental region.
Use of prescribed oral antispasmodic drugs, antibiotics, and corticosteroids in the days prior to drug instillation into the gland is often beneficial to decrease spasm in the excretory ducts, which promotes drainage of saliva or purulent contents and allows for external massage maneuvers to be effective and less painful. In this series, we used dexamethasone 1mL in each session, which was repeated weekly based on patient response and course. If saliva with purulent contents is found, an antibiotic solution may be used; an inexpensive and effective resource is the use of commercial ear drops containing ciprofloxacine or ofloxacine associated to hydrocortisone.
This was an observational study. Quantitative and qualitative variables were considered. A Student's t test was used for quantitative variables, and a Chi-square test was used for quantitative variables. A value of p<0.05 was considered statistically significant, and SPSS version 17 was used for statistical analysis.
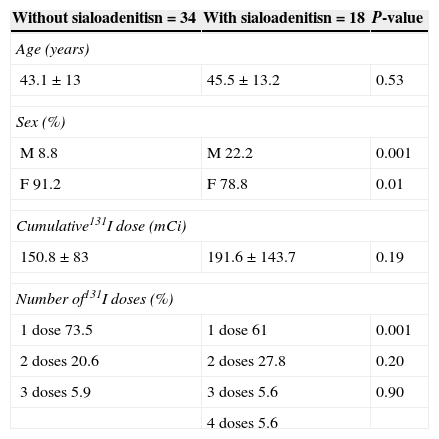
ResultsEighteen of the 52 patients experienced sialoadenitis (34.62%) 5±3 months after administration of 131I, which was considered a late effect of radioiodine. When asked if they had experienced parotiditis, 40 subjects (77%) admitted that they had suffered the disease and 12 patients (23%) denied prior parotiditis. No significant age difference was found between patients with and without sialoadenitis (45±13.2 vs 43.1±13 years, p=0.53); as regards sex, a significantly higher proportion of males was found in the group with sialoadenitis (22.2%) as compared to the group with no sialoadenitis (22.2% vs 8.8%, p=0.001). There was no difference between the groups in cumulative radioiodine dose (p=0.19). As regards the number of ablative doses, the proportion of patients who received a single dose of 131I was significantly higher in the group without sialoadenitis (61%; p=0.001), as shown in Table 1.
Characteristics of patients with or without sialoadenitis after treatment with radioiodine.
| Without sialoadenitisn=34 | With sialoadenitisn=18 | P-value |
|---|---|---|
| Age (years) | ||
| 43.1±13 | 45.5±13.2 | 0.53 |
| Sex (%) | ||
| M 8.8 | M 22.2 | 0.001 |
| F 91.2 | F 78.8 | 0.01 |
| Cumulative131I dose (mCi) | ||
| 150.8±83 | 191.6±143.7 | 0.19 |
| Number of131I doses (%) | ||
| 1 dose 73.5 | 1 dose 61 | 0.001 |
| 2 doses 20.6 | 2 doses 27.8 | 0.20 |
| 3 doses 5.9 | 3 doses 5.6 | 0.90 |
| 4 doses 5.6 | ||
M, male; F, female.
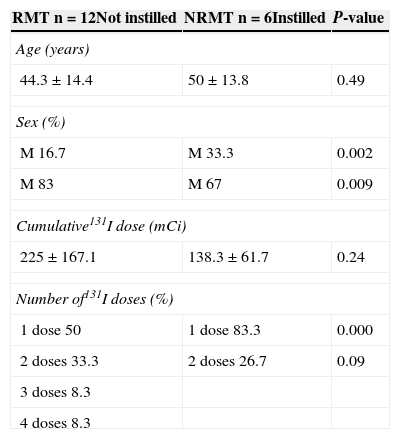
Patients were divided into a RMT group (n=12, 66.67%) and a NRMT group (n=6, 33.33%) based on the clinical course of sialoadenitis. There were no significant age differences (RMT 44.3±14.4 vs NRMT 50±13.8 years; p=0.49); the proportion of males was greater in the NRMT vs the RMT group (p=0.002), while the proportion of females was greater in the RMT vs the NRMT group (p=0.009). Cumulative radioiodine doses did not differ between the groups (RMT 225±167.1 vs NRMT 138.3±61.7mCi; p=0.24). As regards the number of ablative doses, the proportion of patients who received a single dose of 131I was significantly higher in the NRMT group vs the RMT group (p=0.0001), as shown in Table 2.
Characteristics of patients with RMT and NRMT.
| RMT n=12Not instilled | NRMT n=6Instilled | P-value |
|---|---|---|
| Age (years) | ||
| 44.3±14.4 | 50±13.8 | 0.49 |
| Sex (%) | ||
| M 16.7 | M 33.3 | 0.002 |
| M 83 | M 67 | 0.009 |
| Cumulative131I dose (mCi) | ||
| 225±167.1 | 138.3±61.7 | 0.24 |
| Number of131I doses (%) | ||
| 1 dose 50 | 1 dose 83.3 | 0.000 |
| 2 doses 33.3 | 2 doses 26.7 | 0.09 |
| 3 doses 8.3 | ||
| 4 doses 8.3 | ||
M, male; F, female; RMT, patients responding to medical treatment; NRMT, patients not responding to medical treatment.
All six NRMT patients underwent instillation of corticosteroids into the Stensen duct, which resulted in symptom improvement in all of them.
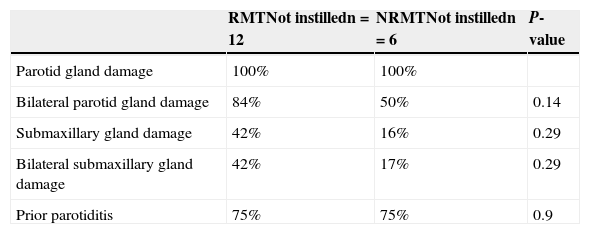
Table 3 shows the greater frequency of scintigraphic damage in parotid glands as compared to the submaxillary glands in the RMT and NRMT groups.
Characteristics of 99Tc scintigraphy of salivary glands in patients with and without response to standard treatment.
| RMTNot instilledn=12 | NRMTNot instilledn=6 | P-value | |
|---|---|---|---|
| Parotid gland damage | 100% | 100% | |
| Bilateral parotid gland damage | 84% | 50% | 0.14 |
| Submaxillary gland damage | 42% | 16% | 0.29 |
| Bilateral submaxillary gland damage | 42% | 17% | 0.29 |
| Prior parotiditis | 75% | 75% | 0.9 |
RMT, patients with response to standard medical treatment; NRMT, patients with no response to standard medical treatment.
Table 3 shows the greater frequency of scintigraphic damage in parotid glands as compared to the submaxillary glands in the RMT and NRMT groups.
DiscussionEmpirical doses for ablation therapy with 131I range from 30 and 150mCi by treatment. However, some patients receive repeated radioiodine doses due to recurrent or persistent disease.
In our population, sialoadenitis occurred after ablation therapy in 34.62% of patients, which agrees with data reported by Grewal et al.22
It is estimated that 24% of the radioiodine dose administered is excreted in saliva, where radioiodine levels are from 20 to 100 times higher than those found in plasma. As the result of exposure to this radiation, cells of gland parenchyma, as well as those of ductal mucosa, experience acute and chronic inflammatory changes. In our sample, in agreement with the literature, parotid glands were more susceptible than submaxillary glands because of their serous structure.2,11,23
Inflammation of ductal mucosa results in stenosis and presence of mucoid saliva, which contribute to duct obstruction and salivary stasis. As a consequence, patients experience pain, swelling, and xerostomia, which are characteristics of the sialoadenitis induced by 131I.16
Our study showed damage evaluable by scintigraphy in patients receiving both one or more ablative doses and in both the RMT and NRMT groups.
Of subjects who experienced sialoadenitis, 61% received a single dose of 131I, which would suggest an individual sensitivity to radiation. The lack of RMT and the decision for intraductal instillation therapy were independent from the number of doses and cumulative dose(s).
Patient age, prior history of parotiditis, and cumulative dose of 131I were not factors influencing treatment response, and only male sex marked a difference between both groups, being a predictor of no response to SMT.
Sialoendoscopic procedures were shown to be helpful for improving symptoms of sialoadenitis in patients refractory to SMT. Nahlieli et al. showed that therapeutic sialoendoscopy provided effective and sustained symptom improvement in most patients.10,15,17–22,24 After the failure of SMT, and since sialoendoscopy was not available at our center, intraductal instillation of corticosteroids was used. Favorable results were found in all patients, and the procedure caused no complications, except for the tolerable local discomfort in some cases.
Local symptoms of sialoadenitis may persist over time. Patients may adapt themselves to hyposalivation, but the normal gland function is not recovered. Patients with DTC have a long life expectancy. Complications resulting from use of ablation therapy, ranging from impaired quality of life to potential development of second tumors, as shown by some authors,25–27 should therefore not be underestimated. The current trend in the management of this disease is an individualized approach, to avoid the use of empirical doses.28 Current consensus emphasizes the need for adequate patient staging and adjustment of ablative dose, particularly in low risk subjects. This would allow for adequate selection of the type of patients who would benefit from this therapy and for whom it would not be recommended. The goal should be to administer the lowest dose of radiation possible to avoid the above mentioned risks.
ConclusionsIn the population studied, incidence of radioiodine-induced sialoadenitis is similar to that reported by other authors. None of the characteristics studied (age, cumulative 131I doses, parotid and submaxillary gland involvement) conditioned a different response to SMT, except for the male gender. We think that if symptoms persist for longer than 15 days, corticosteroid instillation into the Stensen duct is advised, because this is a safe, effective procedure that spares salivary glands, unlike other treatment options such as surgical resection.
Conflicts of interestThe authors state that they have no conflicts of interest related to this paper.
Please cite this article as: Geres AE, Szafryk Mereshian P, Fernández S, Rey Caro DG, Castro R, Podio R, et al. Sialoadenitis por radioyodo. Análisis de factores que influyen en la respuesta al tratamiento médico. Endocrinol Nutr. 2015;62:493–498.