Most prognostic studies in differentiated carcinoma have included a high number of papillary carcinomas and few follicular carcinomas, and not all of their conclusions therefore apply to the latter.
ObjectiveTo analyze the prognostic factors of follicular thyroid carcinoma.
Patients and methodsSelection criteria: Patients with histological diagnosis of follicular carcinoma who had undergone potentially curative surgery, had no disseminated disease at diagnosis, and had been followed up for at least 5 years. Study variables: Tumor recurrence was defined as: (1) tumor lesions with cytological analysis suggesting malignancy and/or (2) patients with total thyroidectomy with thyroglobulin levels >2ng/mL. Clinical, therapeutic, and histological parameters were analyzed to assess prognostic factors.
ResultsRecurrence was found in 25 (38%) of the 66 study patients during a follow-up period of 99±38 months. Most patients with recurrence (n=20) had increased Tg levels without anatomical location, and were initially treated with radioactive I131. In the remaining five cases, surgical excision of the lesion was performed, and three patients required surgery during the follow-up period. Two patients died due to the disease (3%), and two other patients (3%) currently have distant metastases. Mean disease-free interval was 154±14 months, and rates of disease-free patients at 5, 10, 15, and 20 years were 71, 58, 58, and 58% respectively. Clinical factors influencing recurrence included (1) age (p=0.0035), (2) sex (p=0.0114), and (3) cervical pain (p=0.0026). Histological/surgical factors associated with recurrence included (1) infiltration into neighboring structures (p=0.0000), (2) type of carcinoma (p=0.0000), (3) size (p=0.0162), (4) vascular invasion (p=0.0085), and (5) adenopathies (p=0.046). In the multivariate study, cervical pain (p=0.018) and extrathyroid invasion (p=0.045) continued to be significant factors.
ConclusionsIn follicular carcinoma, rates of disease-free patients are 71% at 5 years and 58% at 10 years, and the main predictive factors are presence of local clinical symptoms and infiltration into neighboring structures.
La mayoría de los estudios pronósticos en el carcinoma diferenciado incluyen un alto número de carcinomas papilares y pocos foliculares, por lo que no todas sus conclusiones son aplicables a este último.
ObjetivoAnalizar los factores pronósticos, tanto clínicos, histológicos como terapéuticos, del carcinoma folicular de tiroides.
Pacientes y métodosCriterios de selección: pacientes con el diagnóstico histológico de carcinoma folicular, sin enfermedad diseminada al diagnóstico, con cirugía potencialmente curativa, y con un seguimiento mínimo de 5 años. Variables de estudio: se consideró recidiva del tumor: a) lesiones tumorales con citología sospechosa de malignidad; y/o b) el aumento de los niveles de tiroglobulina mayor de 2ng/ml en pacientes con tiroidectomía total. Para valorar los factores pronósticos se analizan variables clínicas, terapéuticas e histológicas. Estadística: curvas de supervivencia aplicando el test de Breslow. Modelo de regresión de Cox.
ResultadosSe han presentado 25 recidivas (38%) en los 66 pacientes estudiados. La mayoría eran recidivas analíticas (n=20) que fueron tratadas con I-131. En los casos restantes (n=5) se realizó exéresis de la lesión localizada y posteriormente se aplicó I131. Actualmente, dos casos (3%) presentan metástasis a distancia, y otros dos (3%) han sido éxitus por evolución de la enfermedad. El tiempo medio libre de enfermedad fue de 154±14 meses, siendo las tasas de pacientes libres de enfermedad a los 5, 10, 15 y 20 años del 71, 58, 58 y 58% respectivamente. Los factores que influyen en la recidiva son: 1) la edad (p=0,0035); 2) el sexo (p=0,0114); 3) la clínica local (p=0,0026); 4) la infiltración de estructuras vecinas (p=0,0000); 5) el tipo de carcinoma (p=0,0000); 6) el tamaño (p=0,0162); 7) la invasión vascular (p=0,0085); y 8) las adenopatías (p=0,046). En el estudio multivariante persisten la clínica local (p=0,018) y la infiltración de estructuras (p=0,045).
ConclusionesEn el carcinoma folicular los principales factores predictivos son la presencia de clínica local al diagnóstico y la infiltración de estructuras vecinas.
Differentiated thyroid carcinoma (DTC) is the most common thyroid malignancy.1 Most series analyze the different subtypes of DTC together. However, papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) have a different prognosis.1,2 Thus, FTC is more aggressive, although less common (10–25% of differentiated carcinomas), and its incidence is closely related to iodine deficiency in the population.3–5
Because of the above mentioned infrequency and heterogeneity of FTC, joint analysis with PTC makes it difficult to ascertain the true incidence of FTC.3,6 Moreover, the prognostic factors reported in these studies are more predictive for PTC than for FTC.5
Few studies include a sufficient number of patients with FTC for a separate analysis of this condition,7–9 which makes it difficult to detect prognostic predictors or to give recommendations for its management.
The purpose of this study was to analyze the clinical, histological, and therapeutic prognostic factors of FTC.
Patients and methodsSelection criteriaAll patients who underwent surgery from 1980 to 2006 at an endocrine surgery unit were retrospectively analyzed. Their clinical condition was assessed up to December 2011.
Inclusion criteriaPatients who met the following criteria were selected:
- 1
A histological diagnosis of FTC.
- 2
Potentially curative thyroid resection surgery depending on the type of FTC:
- a)
Widely invasive carcinoma: total thyroidectomy and total lymph node dissection in the involved areas
- b)
Minimally invasive carcinoma:
- -
Total thyroidectomy
- -
Partial procedures (hemithyroidectomy or Dunhill procedure [total hemithyroidectomy on the side of the carcinoma and subtotal hemithyroidectomy on the spared side]) in microcarcinomas
- -
- a)
- 3
No disseminated disease at diagnosis (no distant metastases).
- 4
A complete follow-up for at least 5 years, except in patients who died from the disease within this period.
Patients who met any of the following criteria were excluded from the study:
- 1
A histological diagnosis of:
- a)
A follicular variant of papillary carcinoma
- b)
Hürthle cell carcinoma
- a)
- 2
Distant metastases at diagnosis
- 3
Incomplete follow-up in the first 5 years
The series included patients over 31 years, during which time the pathological criteria changed. Moreover, in the 1980s and 1990s there was confusion between FTC and the follicular variant of papillary carcinoma. Therefore, two pathologists reviewed the histological preparations from all patient candidates to study enrollment using current pathological criteria.
Study variablesFTC was considered recurrent when one of the following criteria was met:
- 1
Lesions located by neck examination and/or imaging procedures, regardless of plasma thyroglobulin levels, identified in the cytological study as a suspected malignancy.
- 2
Patients with total thyroidectomy, with plasma thyroglobulin levels >2ng/mL, with or without lesion detection in the physical examination or supplemental imaging tests.
Variables analyzed as prognostic factors included age, sex, symptoms (compression syndromes, hyperthyroidism, and pain-neck discomfort), nodule consistency on examination (hard or elastic), adenopathies in the neck, surgical procedure (total or partial thyroidectomy), histological type based on the degree of capsular invasion (minimally invasive carcinoma: encapsulated tumor showing capsular and/or vascular invasion on microscopic evaluation only; widely invasive carcinoma: tumor showing lack of complete encapsulation, wide areas of invasion by adjacent thyroid tissue and/or extensive blood vessel infiltration),10,11 tumor size (<1cm, 1–4cm, and >4cm), tumor multicentricity, necrosis in some tumor area, vascular invasion by tumor, capsular invasion by tumor, tumor differentiation (well, moderately, or poorly differentiated), and positive adenopathies in the histological study.
Patients with FTC were monitored using baseline thyroglobulin levels under suppression therapy and measurement of thyroglobulin antibodies, stimulated thyroglobulin, neck ultrasound examination, and scan. The intervals between the examinations changed over the study period in line with the various changes recommended by the scientific bodies involved.
StatisticsDescriptive statistics of the characteristics of carcinoma are provided. For the bivariate analysis, survival curves were studied using a Breslow test. To calculate the proportion of the change explained by each variable, a Cox regression model including each significant variable in the bivariate study as a prognostic factor was used. In all cases, differences were considered significant for values of p<0.05.
ResultsDescription of the seriesClinical dataSixty-six patients with a mean age of 41±17 years, the majority of them women (85%; n=56), were selected. The most common complaint was the occurrence of a nodule in the neck (44%; n=29), followed by progressive enlargement of a prior nodule (20%; n=13). On neck examination, a thyroid nodule was palpated in 94% (n=62) of patients. The nodule was of hard consistency in 24 patients (36%). In addition, adenopathies and associated multinodular goiter were palpated in three and two patients respectively. As regards symptoms, 5% (n=3) had hyperthyroidism, 6% (n=4) local pain, 6% (n=4) compression symptoms, and 3% (n=2) dysphonia.
Plain X-rays of the neck and chest taken in all patients were normal, except in five patients (8%) where compression was seen (deviation of tracheal air column). Ultrasound examination performed in all patients confirmed the presence of a non-cystic nodule in all of them, in some cases in the setting of multinodular goiter. Ultrasound confirmed the presence of laterocervical adenopathies in 12 patients. Hormone tests showed that all 66 patients were euthyroid, except for the three patients with hyperthyroidism, and thyroglobulin levels were increased (57±17¿g/L) in 61 patients (92%). A computed tomography scan was performed of one of the intrathoracic goiters with compression symptoms because of the large intrathoracic component detected in X-rays of the neck and chest. Laryngoscopy was performed in the two patients with dysphonia and confirmed the unilateral involvement of a vocal cord. Fine needle aspiration was performed in 29 patients (44%), and Bethesda categories II, III-IV and V were reported in 4, 19, and 6 patients respectively.
Surgical dataSurgery was indicated based on suspected malignancy in 88% of patients (n=58), in 11% (n=7) for goiter, and in one patient for hyperthyroidism. Total thyroidectomy was initially performed in 40 patients (61%) due to highly suspected malignancy (Bethesda V or VI) or bilateral thyroid disease (multinodular goiter), while 24 patients (36%) underwent hemithyroidectomy and a Dunhill procedure was used in the remaining two patients. Total lymph node dissection was performed in all 12 patients with laterocervical adenopathies. All adenopathies were on the same side as the tumor.
Eighteen patients underwent repeat surgery to complete thyroidectomy after the histological confirmation of FTC. In the two patients in whom a Dunhill procedure was performed and in 6 hemithyroidectomies, thyroidectomy was not completed because the minimally invasive tumor was small. After surgery, four patients experienced transient hypoparathyroidism, which became permanent in one of them, and there were four recurrent lesions, one of which became permanent.
Histological dataHistology confirmed the presence of FTC, which was associated with multinodular goiter in 13 patients (20%). The tumor was multicentric in 4 patients (6%), and adenopathies and vascular involvement were found in 2 (3%) and 35 (53%) patients respectively. The tumor was minimally invasive (limited to capsule and/or vascular invasion) in 39 patients (59%) and widely invasive in the remaining 27 patients (41%).
As regards TNM staging,12 56% (n=37) of tumors were stage I, 36% (n=24) stage II, 3% (n=2) stage III, and 5% (n=3) stage IV.
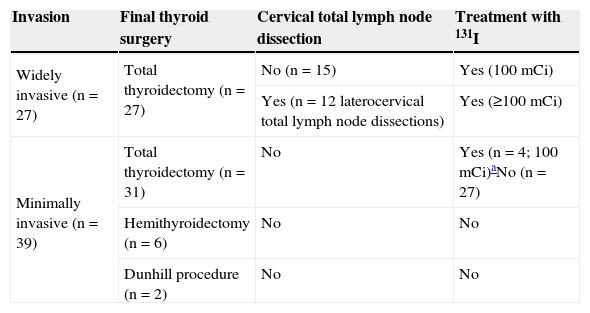
Overall resultsAdjuvant therapy with 131I was administered to 31 patients (47%). Of these, 27 patients had widely invasive tumors, and the remaining 4 patients had minimally invasive tumors greater than 4cm in size (Table 1).
Treatment of patients with FTC.
| Invasion | Final thyroid surgery | Cervical total lymph node dissection | Treatment with 131I |
|---|---|---|---|
| Widely invasive (n=27) | Total thyroidectomy (n=27) | No (n=15) | Yes (100mCi) |
| Yes (n=12 laterocervical total lymph node dissections) | Yes (≥100mCi) | ||
| Minimally invasive (n=39) | Total thyroidectomy (n=31) | No | Yes (n=4; 100mCi)aNo (n=27) |
| Hemithyroidectomy (n=6) | No | No | |
| Dunhill procedure (n=2) | No | No |
Thyroglobulin antibodies were negative in all patients, and thyroglobulin was therefore used as the first tumor marker in all of them. Tumor recurrence occurred in 25 patients (38%) after a mean follow-up of 99±38 months. In all these cases, the recurrence was initially suspected based on a progressive increase in thyroglobulin levels in regular monitoring (37±29¿g/L).
Laboratory recurrences were assessed by thyroid scan and neck ultrasound (according to protocol) to evaluate the treatment to be given. In most patients (n=20), the recurrence was located in the thyroid scan as a slight uptake in the neck, and ultrasound examination did not confirm an organic lesion. In all other patients (n=5), both examinations revealed a lesion in the neck.
How the recurrence was treated depended on its location and size. Thus, the 20 patients with no located lesion were treated with radioactive 131I. In the remaining five patients, the recurrent lesion or adenopathic masses were resected, and 131I was subsequently administered. 131I dosage ranged from 100 to 200mCi depending on the extent of the disease and the risk (the dosagee was individualized). Three patients required two surgical procedures during the course of their disease.
Two patients (3%) currently have distant metastases, and another two (3%) died from the disease. All four patients had widely invasive carcinomas with nodal involvement at the start of the disease, and therefore required the most aggressive surgery at treatment start (total thyroidectomy plus total dissection of laterocervical lymph nodes) and higher initial 131I doses (from 150 to 200mCi).
The mean disease-free time was 154±14 months, with disease-free intervals of 5 years in 71%, 10 years in 58%, 15 years in 58%, and 20 years in 58% of patients. No case of tumor recurrence was detected 9 years or more after surgery.
The TNM has a good prognostic correlation to the disease-free rate. Thus, 5-year disease-free rates were 81% in stage I, 71% in stage II, 50% in stage III, and 0% in stage IV. The corresponding 10-year disease-free rates were 69%, 54%, 50%, and 0% respectively (p=0.009).
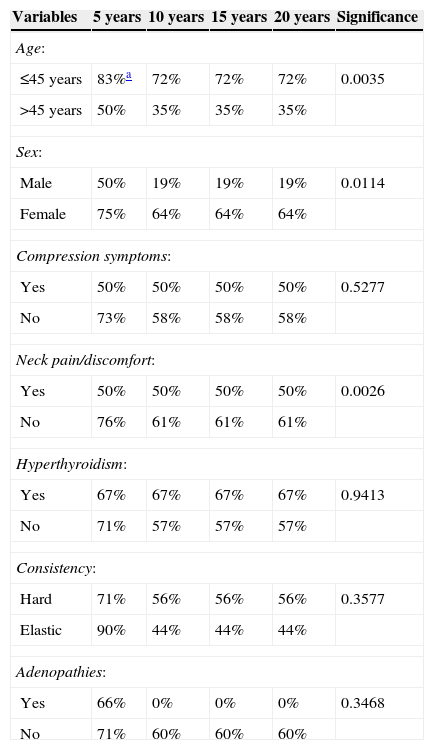
Clinical prognostic factorsAn analysis of the disease-free interval curves, as shown in Table 2, found that the clinical factors influencing disease recurrence include: (1) age (p=0.0035), (2) sex (p=0.0114), and (3) neck pain or discomfort (p=0.0026).
Analysis of the impact of clinical factors on rates of disease-free patients in FTC.
| Variables | 5 years | 10 years | 15 years | 20 years | Significance |
|---|---|---|---|---|---|
| Age: | |||||
| ≤45 years | 83%a | 72% | 72% | 72% | 0.0035 |
| >45 years | 50% | 35% | 35% | 35% | |
| Sex: | |||||
| Male | 50% | 19% | 19% | 19% | 0.0114 |
| Female | 75% | 64% | 64% | 64% | |
| Compression symptoms: | |||||
| Yes | 50% | 50% | 50% | 50% | 0.5277 |
| No | 73% | 58% | 58% | 58% | |
| Neck pain/discomfort: | |||||
| Yes | 50% | 50% | 50% | 50% | 0.0026 |
| No | 76% | 61% | 61% | 61% | |
| Hyperthyroidism: | |||||
| Yes | 67% | 67% | 67% | 67% | 0.9413 |
| No | 71% | 57% | 57% | 57% | |
| Consistency: | |||||
| Hard | 71% | 56% | 56% | 56% | 0.3577 |
| Elastic | 90% | 44% | 44% | 44% | |
| Adenopathies: | |||||
| Yes | 66% | 0% | 0% | 0% | 0.3468 |
| No | 71% | 60% | 60% | 60% | |
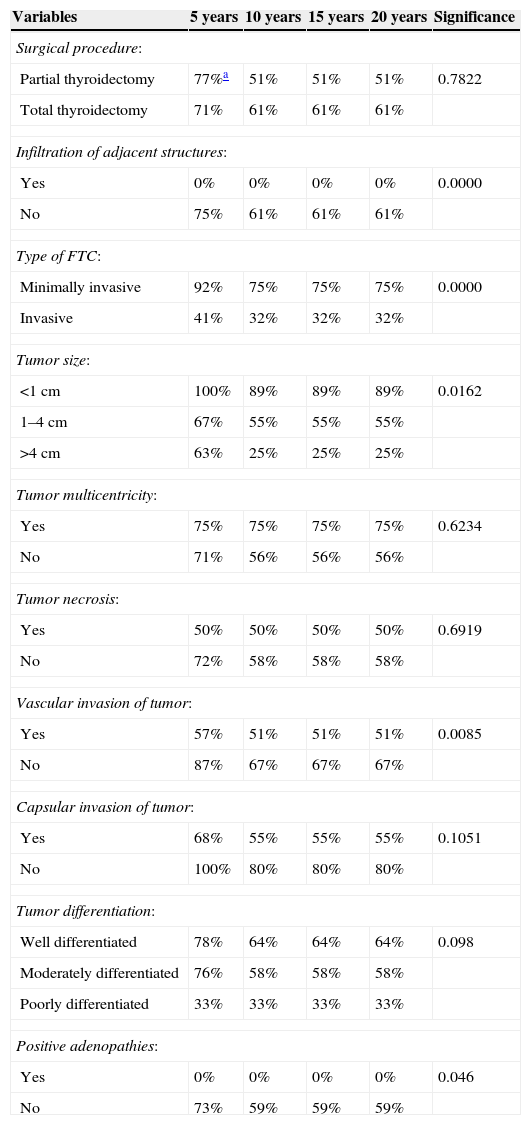
Table 3 shows the surgical and histological factors with an impact on disease recurrence, including: (1) infiltration of adjacent structures at diagnosis (p=0.0000), (2) type of FTC (p=0.0000), (3) tumor size (p=0.0162), (4) vascular invasion (p=0.0085), and (5) positive adenopathies (p=0.046).
Analysis of the impact of surgical and histological factors on rates of disease-free patients in FTC.
| Variables | 5 years | 10 years | 15 years | 20 years | Significance |
|---|---|---|---|---|---|
| Surgical procedure: | |||||
| Partial thyroidectomy | 77%a | 51% | 51% | 51% | 0.7822 |
| Total thyroidectomy | 71% | 61% | 61% | 61% | |
| Infiltration of adjacent structures: | |||||
| Yes | 0% | 0% | 0% | 0% | 0.0000 |
| No | 75% | 61% | 61% | 61% | |
| Type of FTC: | |||||
| Minimally invasive | 92% | 75% | 75% | 75% | 0.0000 |
| Invasive | 41% | 32% | 32% | 32% | |
| Tumor size: | |||||
| <1cm | 100% | 89% | 89% | 89% | 0.0162 |
| 1–4cm | 67% | 55% | 55% | 55% | |
| >4cm | 63% | 25% | 25% | 25% | |
| Tumor multicentricity: | |||||
| Yes | 75% | 75% | 75% | 75% | 0.6234 |
| No | 71% | 56% | 56% | 56% | |
| Tumor necrosis: | |||||
| Yes | 50% | 50% | 50% | 50% | 0.6919 |
| No | 72% | 58% | 58% | 58% | |
| Vascular invasion of tumor: | |||||
| Yes | 57% | 51% | 51% | 51% | 0.0085 |
| No | 87% | 67% | 67% | 67% | |
| Capsular invasion of tumor: | |||||
| Yes | 68% | 55% | 55% | 55% | 0.1051 |
| No | 100% | 80% | 80% | 80% | |
| Tumor differentiation: | |||||
| Well differentiated | 78% | 64% | 64% | 64% | 0.098 |
| Moderately differentiated | 76% | 58% | 58% | 58% | |
| Poorly differentiated | 33% | 33% | 33% | 33% | |
| Positive adenopathies: | |||||
| Yes | 0% | 0% | 0% | 0% | 0.046 |
| No | 73% | 59% | 59% | 59% | |
In the Cox regression study, the only independent risk factors found were neck pain or discomfort (p=0.018) and infiltration of adjacent structures (p=0.045), while sex was borderline significant (p=0.053).
DiscussionFTC is more aggressive than PTC.3,4 Thus, the recurrence rate is quite high (15–40%) in different series,6,13 including ours, with a mean follow-up of 5–10 years. However, long-term survival is high with adequate follow-up and the early treatment of its recurrence, with a disease-related mortality rate of only 3–6%.6
Surgery is the procedure of choice, and although the extent of surgery is controversial, total thyroidectomy is the most currently accepted option. Since thyroidectomy is more radical (tumors are often bilateral and multifocal), it allows for subsequent ablation with 131I, and allows for the use of thyroglobulin and an iodine scan to detect any recurrence.4 Thus, serum Tg levels after total thyroidectomy are an excellent prognostic marker of recurrent or residual disease. These are in many cases the first indication of disease recurrence, and allow for early and successful treatment.4,14 The only counter argument comes from the morbidity associated with total thyroidectomy,15 although this may be performed with low morbidity rates at units experienced in endocrine surgery.16 The main problem, however, is that no preoperative diagnosis of malignancy is available in most cases, and hemithyroidectomy is therefore usually performed.17,18 In such cases, after final histological diagnosis, the completion of thyroidectomy, except when the tumor is minimally invasive or a microcarcinoma, improves the results,6,13,14 as is shown in our series.
Routine lymph node dissection is not indicated if no adenopathies are found.19 Adjuvant ablation therapy with 131I is effective, because it destroys remnant thyroid tissue and undetected metastases and decreases the risk of long-term recurrence.15,20 All of this, together with early repeat surgery when ever there is a recurrence, increases the chances of survival. External radiation therapy is only indicated in patients with unresectable disease or subject to palliative surgery with no 131I uptake.19 Finally, patients should receive an adequate dose of thyroid hormone to suppress TSH production, because such suppression appears to decrease the recurrence rate.19 In recent years, the use of tyrosine kinase inhibitors in advanced cases has provided what appear to be encouraging results, at least as regards slowing the course of the disease, but as yet few data are available.21,22
Some studies have examined the risk factors influencing prognosis in patients with FTC.6,13,15,23,24 The main problem is that these were usually small series, with great variability in patient characteristics and in the detected factors. In principle, the most common factors found to be independent predictors of postoperative recurrence and survival time include the degree of vascular and capsular invasion.6 Other frequently cited factors include age (a worse prognosis the older the age), male sex, extrathyroid extension, the presence of adenopathies, tumor multifocality, and an aneuploid DNA pattern.4,19 Other factors that may be helpful for predicting tumor aggressiveness include the degree of radioactive iodine uptake, adenylate cyclase response to TSH, and the expression of epidermal growth factor receptors.19,25
Age older than 45 years is a poor prognostic factor.6,25,26 In this regard, Hundahl et al.,26 in their analysis of 53,856 patients treated in the US for all thyroid carcinomas, not only FTC, suggest that young age is a favorable prognostic factor for all types of thyroid tumors. In specific studies on FTCs, age is also usually a prognostic factor.6,7,27,28 Thus, Lo et al.7 confirmed that age is an independent risk factor, as did Rao et al.27 and Shaha et al.28. In our series, male sex, which was borderline significant in Cox analysis, was also a poor prognostic factor.
The associated clinical signs and symptoms usually suggested advanced disease.4 Thus, neck pain or discomfort was a sign of poor prognosis in our series, and continued to be an independent risk factor in Cox multivariate study. Compression signs were not prognostic, however, partly because they depend on the concomitant presence of a large multinodular goiter. It should be borne in mind that multinodular goiter is often associated with FTC.3,19,28
One independent risk factor is the involvement of adjacent neck structures at diagnosis, because this also suggests local tumor extension. Thus, Chow et al.29 showed that extrathyroid extension is an independent risk factor for poor prognosis, increasing the risk of a recurrence almost 4-fold.
In addition, it is important to differentiate two forms based on the invasion pattern4,6 (minimally and highly invasive tumors), which have prognostic significance as shown by our series. Several authors6,29–31 have shown that minimally invasive tumors have a better prognosis as compared to widely invasive tumors. Thus, Collini et al.32 reviewed FTCs and noted that minimally invasive FTCs had a better prognosis than invasive tumors, which also occur at an older age, are larger in size, show a greater and quicker growth in thyroid tissue, and cause distant metastases more frequently. FTCs may also be divided based on their growth patterns into well, moderately, or poorly differentiated, which showed in our series a better prognosis, but was not statistically significant. By contrast, other authors24,33 reported that poorly differentiated tumors had higher recurrence rates and decreased survival. On the other hand, multicentricity and nodal involvement are less common as compared to PTC.4
Tumor size is one of the most important prognostic factors.34 It was a predictor in our series, but not in the multivariate study. Rao et al.27 retrospectively analyzed 198 FTCs and concluded that tumors less than 5cm have a low risk, and Shaha et al.28 analyzed 228 FTCs and reported that tumor size greater than 4cm is among the main prognostic factors.
Vascular invasion is a poor prognostic factor,4,6 so that vascular extension (more than four vessels involved) is a risk factor for recurrence.32 Finally, the presence of positive adenopathies has also been reported to be a poor prognostic factor, although this poor prognosis was not verified in most studies,35 and did not persist in the multivariate analysis in our study.
Few data are currently available about mutations in FTC that may have prognostic implications and could allow us to use more aggressive treatments after diagnosis. The B-RAF mutation appears to have prognostic significance in papillary carcinoma, but few data are available for FTC.36,37
In conclusion, it may be stated that the main factors predicting for FTC recurrence are age over 45 years, male sex, local clinical signs at diagnosis, the infiltration of adjacent structures, the type of carcinoma, size, capsular or vascular invasion, and the presence of adenopathies. Final independent factors include local clinical signs and the infiltration of adjacent structures.
In the absence of prospective studies, prohibited by the low number of patients and the heterogeneity of FTC, it is difficult to draw conclusions regarding the best treatment. Therefore, if we really want to have conclusive data about this tumor, multicenter studies should be conducted to recruit a prospective patient sample large enough to allow for achieving valid results.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Ríos A, Rodríguez JM, Ferri B, Martínez-Barba E, Torregrosa NM, Parrilla P. Factores pronósticos del carcinoma folicular de tiroides. Endocrinol Nutr. 2015;62:11–18.