A 33-year-old primigravida was admitted to our center for hyponatremic coma 7 days after delivery of a healthy infant. She had no remarkable personal or family history, and was admitted to a private hospital for delivery after an uneventful full term pregnancy. High blood pressure was found upon admission, and a cesarean section was therefore decided, which was complicated by heavy bleeding due to a right uterine ligament tear. Subsequent laboratory tests showed AST levels of 100U/L (5–40), anemia (hemoglobin 6.5g/dL [12–16]), thrombocytopenia (21×10E3/μL, 150–450), and proteinuria in the nephrotic syndrome range. Four RBC units and seven platelet units were transfused, and urapidil, labetalol 200mg/6h, and methyldopa 500mg/6h were infused due to sustained HBP. A single dose of cabergoline 1mg was administered to inhibit lactation on the patient decision.
Seven days after birth, patient reported severe asthenia and headache. Physical examination showed no changes and BP values of 150–155/95–100mmHg. A complete blood count revealed persistent anemia. A few hours later the patient experienced a tonic–clonic seizure followed by coma and was intubated. She remained afebrile and hemodynamically stable, with a BP of 135/80mmHg and a HR of 67bpm. Laboratory tests revealed worsening of anemia, blood glucose of 73mg/dL, CPK of 1710U/L related to the seizure, and Na of 103mmol/L. Patient was transferred to the ICU of our center because of the need for imaging tests.
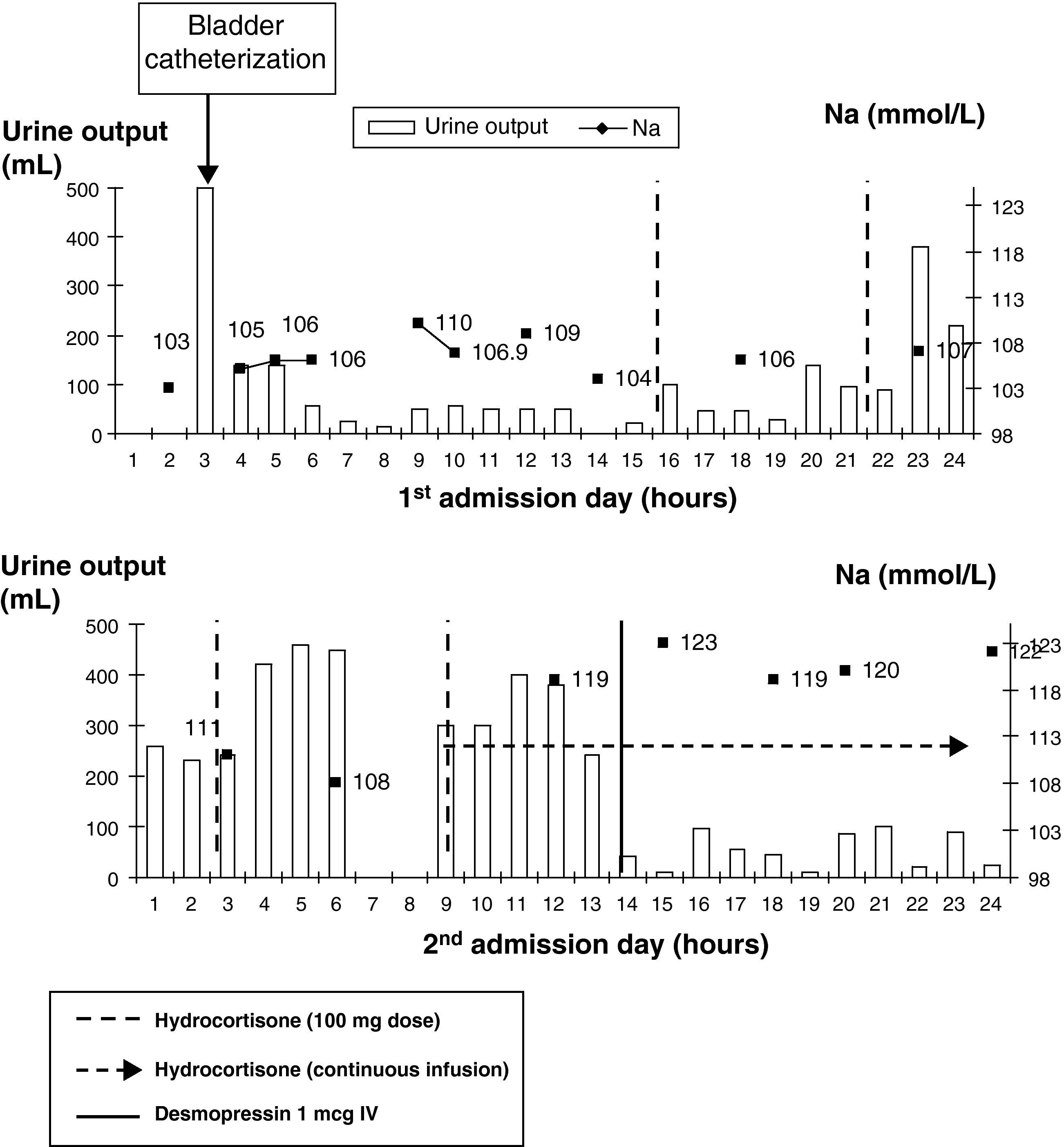
Upon arrival to the ICU, sodium levels of 104mmol/L (135–145) were found, as well as glucose 78mg/dL (60–100), urea 19mg/dL (15–50), creatinine 0.57mg/dL (0.5–1.2), and serum osmolality of 224mOsm/kg. Urinary analysis showed Na 41mmol/L and osmolality of 384mOsm/kg. There was persistent normocytic and normochromic anemia with hemoglobin 8.2g/dL, and slightly elevated transaminase levels. Chest X-rays and a CT scan of the head showed no changes, and diffuse brain involvement was found in an electroencephalogram. Isotonic saline, 2000mL over 5h, was started at the ICU with no improvement. Urine output during the first admission day ranged from 15 to 50mL/h. Hormone tests requested provided the following values: cortisol 1.6ng/dL (5–25), which increased to 19.1ng/dL (30′) and 18.7ng/dL (60′) after short stimulation with intravenous ACTH 250mcg, TSH 0.56mU/L (0.34–5.6) and free T4 0.57ng/dL (0.58–1.64). Adrenal insufficiency was suspected, and replacement was therefore started with hydrocortisone 100mg every 6h. Urine output increased 2h after each dose (145mL after the first dose, 470mL after the second, and 880mL after the third), but urine output again decreased a few hours after each dose, and a continuous hydrocortisone infusion was therefore started.
On the second day, patient had normal Na at 6:00h. At 9:00h, repeat hormone tests showed a free T3 value of 1.8pg/mL (2.5–3.9) and still decreasing TSH and free T4 levels. At 12:00h, Na level increased to 119mmol/L (11mmol/L in 6h) and urine output to 300–400mL/h, and intravenous desmopressin 1mcg was therefore administered (Fig. 1).
Laboratory test results on the third day included: hemoglobin 8.3g/dL, glucose 112mg/dL, creatinine 0.47mg/dL, uric acid 0.8mg/dL (2.5–6), Na 120mmol/L, K 4.2mmol/L, LDH 869U/L, and normalization of transaminase levels. Hormone tests showed the following: prolactin 2ng/mL, undetectable LH and FSH, cortisol 41.4ng/dL, TSH 0.34mU/L, free T3 1.87pg/mL, and free T4 0.52ng/dL. During the third and fourth days, sodium levels gradually increased to 138mmol/L, with 10mmol/L changes in less than 24h and urine output values higher than 300mL/h. Desmopressin 1mcg was therefore administered again.
Deintubation was decided based on recovery of sodium levels, and patient remained conscious and oriented, with no focal neurological signs or new seizures. MRI showed a pituitary gland of normal shape and size, with peripheral enhancement and no central uptake with gadolinium contrast, consistent with non-hemorrhagic subacute adenohypophyseal ischemia. Sodium levels remained stable, and a gradual improvement occurred in thyroid hormone levels (free T4 0.62ng/dL on the sixth day).
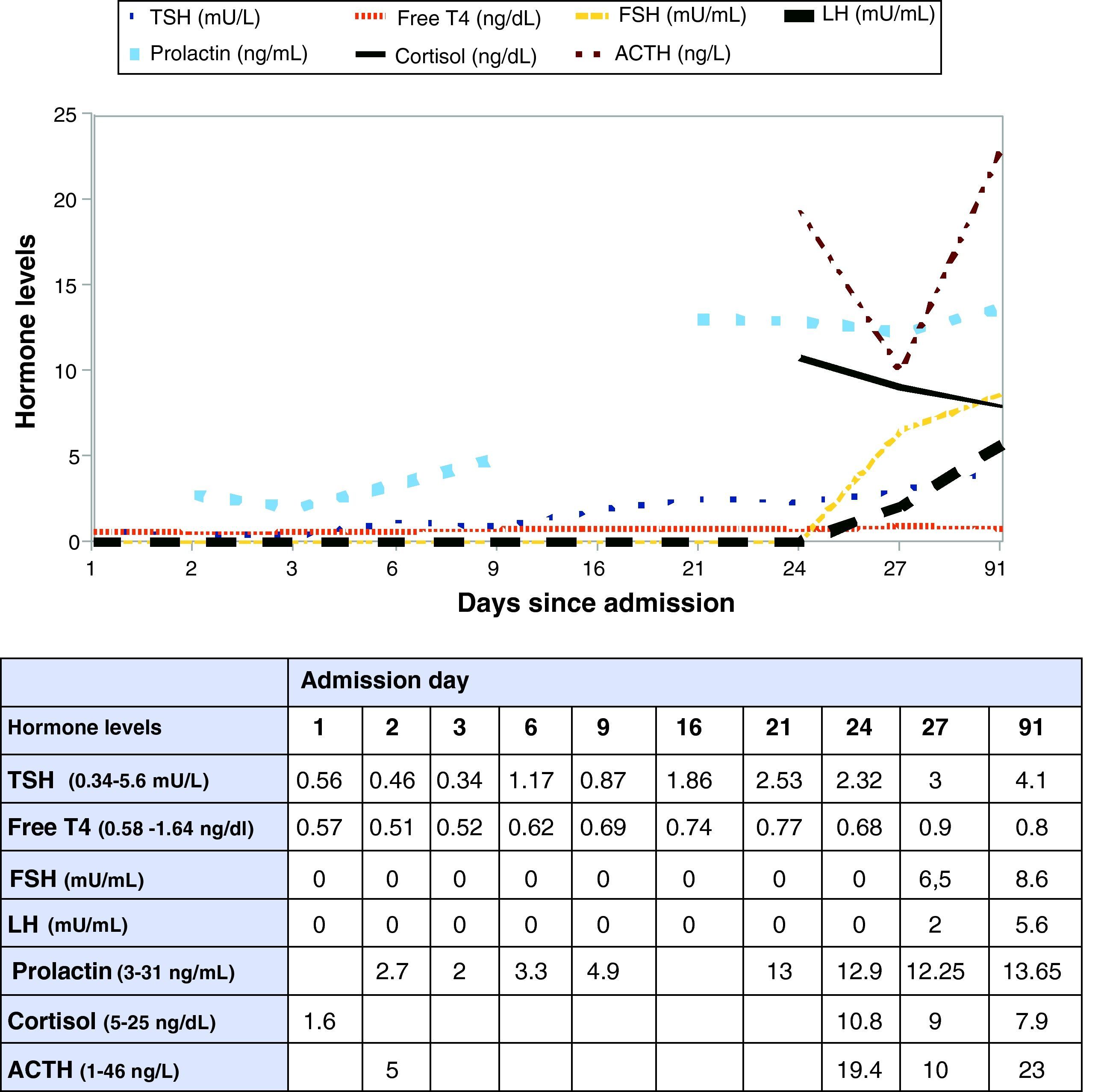
Patient was transferred to the endocrinology ward. Improvement in BP levels and disappearance of proteinuria allowed for tapering of antihypertensive drugs. Based on improved hormone control, decreased ischemia in the control MRI, and negative immune tests, corticosteroid tapering was decided. On hospitalization day 27, hormone tests were again performed before discharge, showing basal cortisol of 9ng/dL, increasing to 14.8ng/dL (30′) and 5.5ng/dL (60′) after short stimulation with intravenous ACTH 250mcg, and improvement in all other parameters. It was therefore decided to prescribe corticosteroid therapy under stress conditions only (Fig. 2).
After discharge, patient did not still require background treatment. Menses returned at 5 months of discharge, and became regular again at 8 months. Partial cortisol response to short ACTH stimulation test persists, while all other hormones have recovered. An ischemic image persisted at 6 months in MRI.
Analysis of data recorded during her stay at the ICU showed that the patient had TSH levels in the low range with inadequate thyroxine levels after pregnancy. Although this was initially attributed to hemodilution, the trend of free T4 to continue decreasing despite increased sodium levels and administration of high glucocorticoid doses led to suspect central hypothyroidism. As regards the corticotropic axis, patient had inappropriately low basal cortisol levels for the stress situation she experienced, with a virtually normal response to the short ACTH stimulation test. This suggested a recent onset central adrenal insufficiency with an almost preserved capacity to respond to exogenous ACTH of the adrenal glands, which had not atrophied yet. In addition, prolactin appeared to be too decreased for a single 1mg dose of cabergoline.
It would have been helpful to perform specific pituitary function stimulation tests, but these were not feasible because of the patient's condition. Thus, we think that the decision to start stress doses of hydrocortisone was adequate, and that thyroxine replacement would also have been appropriate.
Evidence of hypopituitarism combined with a history of obstetric complications with bleeding requiring transfusion and the ischemic image of adenohypophysis in MRI1,2 suggested a Sheehan syndrome (SS) as first diagnostic possibility.
While hyponatremia is the most common electrolyte change in SS, it is usually chronic in nature, and although rare in developed countries, hyponatremic coma is part of the acute presentation of SS.3–7 The mechanisms by which it occurs range from “adequate” vasopressin hypersecretion to cortisol deficiency and, to a lesser extent, volume depletion (due to labor complications) and hypothyroidism (due to impaired free water clearance), mimicking a syndrome of inappropriate antidiuretic hormone secretion, but we should not forget that this condition is diagnosed by exclusion. Because of its pathophysiology, hyponatremia in SS is characterized by the lack of response to isotonic and hypertonic saline infusion until this is not associated to replacement glucocorticoid and thyroxine therapy to decrease compensatory vasopressin hypersecretion.3,6
Although thyroxine was not administered to our patient, an analysis of the flowchart tables (Fig. 1) showed that natremia and urine output did not start to improve until hydrocortisone was started. It is of note that 3% hypertonic saline was not administered at the ICU to a patient with severe (Na level of 115mmol/L or less) symptomatic hyponatremia with seizures and subsequent coma suggesting acute hyponatremic encephalopathy, because women of childbearing age are known to be part of the group at a greatest risk of ischemia, brain herniation with brain stem compression, and death.
Although the fear to use hypertonic saline because of the risk of occurrence of an osmotic demyelination syndrome is understandable, the risk does not lie so much in its use but in its delayed discontinuation. Close monitoring is therefore required to prevent increases in sodium levels greater than 2mmol/L/h, 10mmol/L/24h or 18mmol/L/48h. This would explain desmopressin administration (although this was a debatable treatment) at the ICU when rapid increases were seen in sodium levels associated to urinary frequency.
In addition to the exceptional acute presentation of SS in this patient, a decrease in ischemic surface was seen in MRI during its course, as well as a gradual recovery of thyroid hormones (Fig. 2), TSH, cortisol, and prolactin which allowed for treatment discontinuation. The condition therefore appears to be a transient SS, already reported in the literature, although most recoveries from it were only seen in the gonadotropic and/or lactotropic axes,8,9 and recovery of the corticotropic and somatotropic occurred in a single case.2
Finally, this case emphasizes the significance of laboratory monitoring and especially electrolyte levels in patients with complicated labor, and the need for early recognition of a condition that may be fatal for women.7
Please cite this article as: Currás Freixes M, et al. Coma hiponatrémico posparto. Endocrinol Nutr. 2011;58:372–5.