In 1898, Pearce Bailey reported the first case of pituitary apoplexy (PA); however, it was not until 1950 that Brougham et al. recognized this syndrome in five patients, reviewed the medical literature and coined the term PA.1
PA is a clinical syndrome characterized by headache, vomiting, visual defects, ophthalmoplegia, impaired consciousness and/or meningism due to infarction or bleeding from a pituitary adenoma.1,2 PA is an uncommon but potentially fatal endocrine emergency occurring in patients with pituitary adenomas, particularly non-secreting macroadenomas.3 In 80% of cases, PA is the first symptom of the pituitary adenoma.4,5
The incidence of PA in pituitary adenomas ranges from 2% to 7% in most series.2,6 However, the frequency of subclinical PA, i.e. incidentally detected pituitary hemorrhage and/or asymptomatic infarct, may be as high as 25%.2,5
Changes in visual acuity and field are due to the rapid growth of pituitary adenoma, which extends laterally into the cavernous sinus and compresses the third, fourth, and sixth cranial nerves; or upwards, compressing the optic chiasm.7,8
The case of a patient who attended the emergency room for headache, nausea, and diplopia is reported here. Magnetic resonance imaging (MRI) showed a pituitary macroadenoma with signs of hemorrhage. Transsphenoidal surgery resolved ocular changes.
A 71-year-old male patient with a history of dyslipidemia not treated with drugs attended the emergency room of our hospital complaining of headache, nausea, vomiting, diplopia, and impaired visual field for the previous two weeks. Three months before admission, the patient had experienced self-limited episodes of diplopia. One day before admission, he visited a physician, who requested MRI of the brain (Fig. 1) and referred him to our hospital with a diagnosis of pituitary macroadenoma. At admission, the vital signs of the patient were: BP, 160/100mmHg; HR, 56 beats per minute; RR, 19 breathings per minute; and oral temperature, 38°C. Physical examination revealed palsy of the third, fourth, and sixth right cranial nerves, a miotic right pupil with no photomotor reflex, and bitemporal hemianopsia. No meningeal signs were seen. Laboratory test results were normal, an electrocardiogram showed first degree atrioventricular block, and chest X-rays were normal.
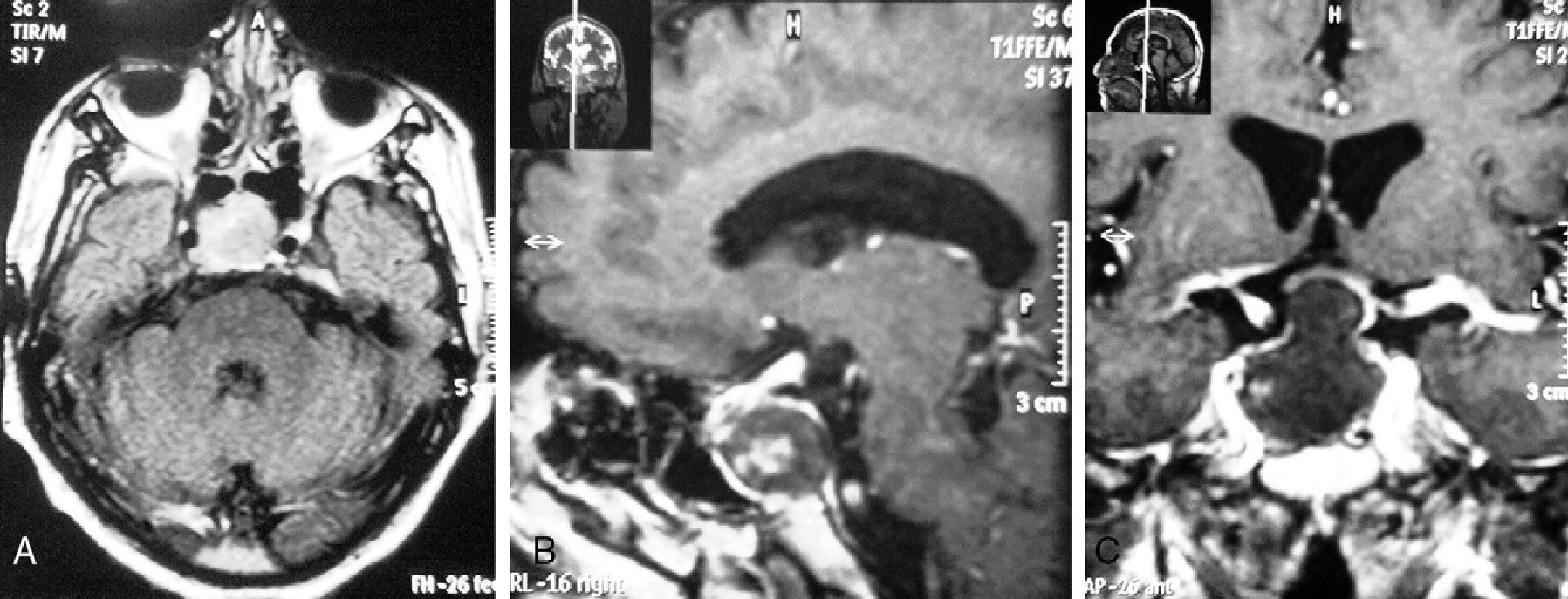
(A) T1-weighted MRI without contrast (cross section). A heterogeneous and hyperintense sellar and suprasellar tumor, approximately 20mm in diameter, with hypointense contents and compromising the right cavernous sinus is seen.
(B) T2-weighted MRI with gadolinium (sagittal section at cerebral falx level). The image shows a tumor with hyperintense margins and heterogeneous contents, approximately 20mm in diameter in the sellar region, that compresses and displaces the sella turcica. (C) T1-weighted MRI with gadolinium (coronal section at sella turcica level). The image shows high contrast uptake by a tumor with hyperintense margins and heterogeneous contents that compresses the optic chiasm and invades both cavernous sinuses, predominately on the right side.
The patient was admitted to hospital with the diagnoses of intracranial hypertension syndrome and pituitary apoplexy. Pituitary hormone levels were as follows: prolactin 4.6ng/mL (NR, 3.1–16.5), TSH 3.44μU/mL (NR, 0.3–5), free thyroxine 0.97ng/dL (NR, 0.8–2), FSH 1.7mU/mL (NR, 2–11), LH 1.1mU/mL (NR, 0.4–5.7), total testosterone 10pg/mL (NR, 181–772), ACTH 16.7pg/mL (NR, 5–63), and basal cortisol 11.8μg/dL (NR, 6.2–26).
The patient underwent transsphenoidal surgery with resection of the pituitary adenoma. Postoperatively, the patient experienced diabetes insipidus, adrenal insufficiency, and acute anemia due to nose bleeding. Pathological examination supported a diagnosis of non-secreting pituitary macroadenoma. Pituitary hormone levels measured after surgery were prolactin 1.4ng/mL, free thyroxine 0.22ng/dL, and basal cortisol <1μg/dL.
The patient was discharged on replacement therapy consisting of prednisone 7.5mg/day and levothyroxine 75μg/dL. At three months he had recovered motility in his right eye and had no discomfort.
The most frequent presenting symptoms of PA include headache (92%), nausea and vomiting (54%), decreased visual field (56%), ocular palsy (54%), bitemporal hemianopsia (34%), and impaired consciousness (42%).9,10 Hypopituitarism may occur in 50%-87% of cases.11 In these patients, rapid adenoma growth causes a sudden increase in intrasellar pressure which results in ischemic necrosis of various portions of the anterior pituitary gland, limiting the chances of hormone function recovery.12 ACTH deficiency, reported in 70% of cases, is the most important hormone deficiency. TSH and gonadotrophin deficiency have also been reported in 50% and 75% of cases respectively.2 At admission, patients with decreased prolactin levels have an increased intrasellar pressure and the least chance of recovering hormone function after surgery.2,12 On the other hand, adrenal insufficiency is the main cause of death.2,11
Visual field defects and decreased visual acuity may be caused by compression of the cranial nerves or the optic chiasm. The rapid growth of pituitary adenoma may compress the cranial nerves against the wall of cavernous sinus or directly infiltrate the sinus.7,8 Early surgical decompression (within seven days of the start of visual defects) is associated with recovery of vision and endocrine function.2,5–9,13
A differential diagnosis of PA includes the growth of a carotid or basilar aneurysm. In more than 90% of aneurysms of the posterior communicating artery, oculomotor nerve paralysis occurs before symptoms of subarachnoid hemorrhage. Other diagnoses to be considered include intracranial hemorrhage, subarachnoid hemorrhage, bacterial meningitis, cavernous sinus thrombosis, and cerebral infarction.7,14
Factors triggering PA include coronary surgery, major surgery, pregnancy, radiation to the head, anticoagulation, coagulopathy, endocrine stimulation tests,15 high blood pressure, estrogen use, head trauma, or discontinuation of dopamine receptor agonists.2 However, a study found that the use of dopamine receptor agonists could be a protective factor.6 Patients undergoing major surgery may experience blood pressure fluctuations that may trigger PA.2,16 On the other hand, dynamic stimulation tests using GnRH, TRH, or CRH may increase intrasellar pressure and trigger PA.2,16
MRI is the imaging test of choice for diagnosis because it detects 90% of the cases (computed tomography detects 20% of cases only).2,10,13 As regards treatment, glucocorticoid replacement therapy is the most effective medical intervention because secondary adrenal insufficiency is the most significant risk factor for death and complications.2,12 The use of hydrocortisone 100–200mg IV, followed by a continuous IV infusion of 2–4mg per hour is recommended. Due to saturation kinetics of cortisol binding globulin, intermittent IV injections of hydrocortisone are of less value (much of the administered dose is filtered in the urine and therefore not available). The use of dexamethasone is not recommended; however, it could be useful in decreasing edema as a non-surgical approach to PA.2
Transsphenoidal decompression is the treatment of choice, particularly in patients with severe neuro-ocular signs or impaired consciousness.1,2 Diabetes insipidus may occur after surgery in 16% of patients. Other potential complications include cerebrospinal fluid loss, meningitis, and acute adrenal insufficiency.2 On the other hand, the use of glucocorticoids is recommended in patients with hemodynamic instability, decreased consciousness, decreased visual acuity, or severe visual field defects.2,11
Most patients will require some type of hormone replacement therapy during long-term follow-up. Growth hormone deficiency is most common, but patients will also require glucocorticoids (60–80%), thyroid hormone (50–60%), desmopressin (10–25%), and testosterone (60–80%).1,2
As regards visual changes, most patients undergoing surgery experience almost total recovery of vision, which starts to be noticed early after surgery and increases in subsequent weeks,2,7 particularly from ocular paresis and when early pituitary decompression surgery has been performed.1
Please cite this article as: Pinto-Valdivia M, et al. Apoplejía pituitaria. A propósito de un caso. Endocrinol Nutr. 2013;60:37–47.