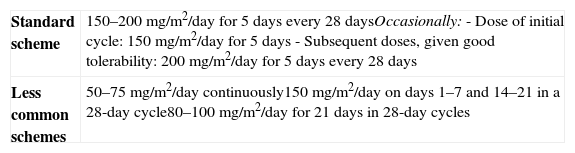Non-functioning pituitary adenomas are the most common pituitary macroadenomas in adults, accounting for approximately 14–28% of all clinically relevant pituitary tumors. They are a heterogeneous group of tumors that cause symptoms by compression and/or hormone deficiencies. The possibility of tumor growth is increased in macroadenomas and solid tumors as compared to microadenomas and cystic tumors. Diagnosis is based on imaging procedures (magnetic resonance imaging), but there are studies reporting promising potential biomarkers. Transsphenoidal surgery remains the first therapeutic option for large tumors with compressive symptoms. There is no evidence that endoscopic procedures improve outcomes, but they decrease morbidity. There is no unanimity in finding prognostic predictors of recurrence. Radiosurgery achieves tumor control and, sometimes, adenoma size reduction. Its adverse effects increase with higher doses and tumor sizes >4cm3. Drug treatment is of little value. In aggressive non-functioning tumors, temozolomide (TMZ) may be used with caution because no controlled studies are available. TMZ achieves tumor control in 38–40% of aggressive non-functioning tumors. The optimal treatment regimen and duration have not been defined yet. Lack of response to TMZ after 3 cycles predicts for treatment resistance, but initial response does not ensure optimal mid or long-term results. O6-methylguanine-DNA methyltransferase expression has a limited predictive value of response to treatment with TMZ in aggressive non-functioning tumors. It should therefore not be a determinant factor in selection of patients to be treated with TMZ.
Los adenomas hipofisarios no funcionantes son los macroadenomas hipofisarios más frecuentes en adultos y representan el 14-28% de todos los tumores hipofisarios clínicamente relevantes. Son un grupo heterogéneo de tumores que causan síntomas por compresión o por déficits hormonales. La posibilidad de crecimiento tumoral aumenta en macroadenomas y tumores sólidos en comparación con microadenomas y tumores quísticos. El diagnóstico se basa en técnicas de imagen (resonancia magnética) pero hay estudios prometedores sobre posibles biomarcadores. La cirugía transesfenoidal sigue siendo la primera opción terapéutica en tumores grandes con síntomas compresivos. No hay evidencia de que la técnica endoscópica mejore los resultados aunque disminuye la morbilidad. No hay unanimidad en encontrar factores pronósticos de recurrencia. La radiocirugía consigue control tumoral y, en ocasiones, reducción de tamaño del adenoma. Sus efectos adversos aumentan con las dosis altas y el tamaño tumoral>4cm3. El tratamiento farmacológico tiene escasa utilidad. En los tumores no funcionantes agresivos se puede emplear temozolomida (TMZ) pero con precaución, porque no hay estudios controlados hasta la actualidad. Consigue control tumoral en el 38-40% de tumores agresivos no funcionantes. La pauta de tratamiento óptima y la duración del mismo están por definir. La falta de respuesta a TMZ tras 3 ciclos predice resistencia al tratamiento, pero la respuesta inicial no asegura resultados óptimos a medio o largo plazo. La expresión de O6-metilguanina-ADN-metiltransferasa tiene escaso valor predictivo de respuesta al tratamiento con TMZ en tumores no funcionantes agresivos. Por eso no debe ser un determinante en la selección de pacientes para tratar con TMZ.
Pituitary adenomas account for less than 10–15% of primary intracranial tumors.1 Non-functioning pituitary adenomas (NFPAs) account for 14–28% of all clinically relevant pituitary adenomas2,3 and for 50% of pituitary macroadenomas.4 They are a heterogeneous group of tumors with clinical and biochemical characteristics different from those of functioning pituitary adenomas. Knowledge of the histological subtype of NFPA helps predict its postoperative course, because some of them appear to show more aggressive behavior. Most NFPAs express gonadotropins or their subunits (α and β) and represent 10% of all pituitary adenomas.5,6 Almost 15% of NFPAs are silent adenomas which can express, but not secrete, other pituitary hormones (ACTH, TSH, PRL, and GH).6 Thirty percent of NFPAs, null cell adenomas, do not express or secrete any hormone.7 Silent subtype 3 adenomas are very rare, have a high recurrence rate, and require electron microscopy for diagnosis. Most of their tumor cells are immunonegative, but a minority variably express several types of hormones (GH, PRL, TSH, α,β-endorphin subunit, and ACTH).8
Etiopathogenesis of non-functioning pituitary adenomasNFPAs usually are benign monoclonal tumors9 consisting of neoplastic epithelial cells of the anterior pituitary gland, most of which are of gonadotropic origin.10 Many genetic changes that may contribute to the development of pituitary adenomas have been reported to date,11–16 but it is difficult to attribute special relevance to any of them. Approximately 10% of NFPAs show mutations in the regulatory GNAS gene, but this is a small proportion as compared to the relatively high frequency (>40%) found in somatotropinomas. Changes in various tumor suppressor gene regions, such as MEG3,10 are common, particularly in patients with invasive NFPAs, but it is not clear whether this contributes to tumor pathogenesis or is only an epiphenomenon. The pituitary tumor transforming gene (PTTG) may be a permissive oncogene in the development of pituitary adenomas.17 While PTTG deletion facilitates pituitary hypoplasia and confers some protection against the development of pituitary tumors,18 PTTG overexpression, which has been detected in pituitary adenomas,19 is associated with increased aneuploidy and angiogenesis.20 Deficiency of the pituitary tumor apoptosis gene has also been reported in some pituitary tumors. This may modify the normal mechanisms of apoptosis directed to the elimination of cells with potentially tumorigenic genetic changes. The expansion of an abnormal cell clone to form a final tumor could thus be promoted.
Hypothalamic releasing factors and growth factors locally produced by tumor cells themselves appear to act as progression factors rather than as initiators of tumorigenesis. Interleukins synthesized in the pituitary gland may also have some effect on tumor behavior. Interleukin-6 may stimulate adenoma progression21 by being able to inhibit the growth of normal pituitary cells and stimulate the growth of tumor cells.22
Unfortunately, no single molecular change which serves as a therapeutic target or helps predict tumor behavior and potential recurrence has been detected yet in NFPAs. The mechanisms through which some functioning adenomas are clinically silent are not known either. Tpit, a tissue-specific regulator of pro-opiomelanocortin expression, has been detected in corticotroph adenomas and some, but not all, authors have found lower levels when corticotroph adenomas are silent. There are no differences in Pit-1 and GHRH receptor mRNA expression in somatotropic, thyrotropic or lactotropic, silent or functioning tumors.
Clinical symptoms and signsNFPAs are diagnosed between 20 and 60 years of age in 78% of cases3 and are somewhat more common in males.23 As they cause no signs or symptoms of hormone hypersecretion, they are often incidentally found in radiographic tests done for other reasons (incidentalomas).3,24 In the most recent series, almost 50% of NFPAs are incidentalomas.25
NFPAs only cause clinical symptoms when their size is able to alter hormone secretion, affect the optic chiasm, increase intracranial pressure, or damage neurological structures. Fifty to 60% of patients with macroadenomas have visual disturbances at diagnosis3,26 and 50–62.1% have headache.3,24 GH and gonadotropin deficiencies are the most commonly found. Cases where uncinate attacks due to tumor extension toward the temporal lobe occur or where personality changes and anosmia due to frontal lobe damage occur are very rare.
After surgery, patients with non-functioning macroadenomas may also experience asthenia and drowsiness leading to a significant decrease in their quality of life.26 Both systems have been related to the presence of hypopituitarism, but sleep disturbances, frequently occurring when suprasellar tumor expansion exists, may also contribute.27
DiagnosisThere are no helpful biomarkers for the diagnosis of NFPAs, but specific molecular structures have recently been isolated from patients with NFPAs by comparing their sera to those of control subjects.28 Mass spectrometry, which makes it possible to detect proteins with a lower molecular weight (<15kDa) than the standard bidimensional genes, has been used for this purpose. The heterogeneity of molecules found in patients with NFPAs may reflect the existence of several tumor subtypes and complicates laboratory diagnosis. The results achieved so far have been encouraging, but further studies recruiting more patients are needed to ascertain the sensitivity and specificity of this technique.
To date, the diagnosis of NFPAs has mainly been based on radiographic tests. Magnetic resonance imaging (MRI) with gadolinium contrast is the procedure of choice. In older series, up to 66.7% of NFPAs were macroadenomas at diagnosis,3 but this proportion has tended to decrease in recent years as the result of the increased use of MRI for the study of various clinical conditions not related to pituitary disease. Although there have been attempts to determine whether the radiographic findings suggesting aggressiveness in MRI correlate to morphological changes in adenomas, the results have not been conclusive.29,30 Nishioka et al., in a retrospective study conducted on 388 patients with NFPAs referred for surgery, found that silent adenomas most commonly showed radiographic changes related to aggressiveness (large size, cavernous sinus invasion, and lobulated suprasellar margins) and that the proliferation indices were higher in non-corticotroph silent adenomas and in giant adenomas of any histological type. They found no relationship between proliferation indices and age, sex, cavernous sinus invasion, or the lobulated suprasellar extension of adenomas. The authors also assessed the possibility of predicting NFPA subtypes based on radiographic findings and concluded that, in 91% of patients, pituitary tumors with no radiographic signs of aggressiveness in patients older than 40 years are gonadotropinomas or null cell adenomas.31
If NFPAs are incidentally detected in the radiographic study, the presence of unsuspected deficiencies or hormone hypersecretion should be ruled out. Most incidentalomas are non-clinically functioning adenomas, but approximately 18% may be functioning, especially prolactinomas or GH-secreting adenomas. Because of this, some authors such as Orija et al. suggest that, in asymptomatic microincidentalomas (<1cm), the measurement of PRL and IGF-1 should suffice, and all other pituitary hormones should only be assessed if hyperfunction is suspected based on the clinical picture of the patient.32 Other authors, however, recommend that the presence of corticotroph adenomas should always be ruled out. All macroincidentalomas should be investigated to rule out pituitary hormone changes and/or visual involvement.32
Natural historyLittle is known about the natural history of NFPAs because most cases are treated with surgery and we do not know what their course would have been. Tumor growth has been reported in 5.8% of patients per year.33 Non-functioning microadenomas usually remain stable, but 35.3% of macroadenomas tend to grow during follow-up,34,35 and when this occurs, half the tumors also show clinical worsening.7 A meta-analysis conducted by Fernández-Balsells et al., examining the incidence of adverse events in patients with untreated NFPAs suggested that solid lesions and those with a size >10mm may most commonly cause complications during follow-up. However, the authors concluded that the grade of evidence found was low, because most studies analyzed were non-comparative cohort studies with significant methodological limitations, and many of them had only a small number of patients.33
When NFPAs are monitored, it should also be borne in mind that spontaneous tumor regression may occur in up to 11% of patients, possibly due to silent ischemia,4 and that after an episode of pituitary apoplexy, new tumor growth may occur in another 11% of patients.36
TreatmentWhen the treatment of NFPAs has to be decided upon, age, size, the presence of visual disturbances, endocrine function, whether or not growth occurs during follow-up, and the natural history of these tumors should all be taken into consideration. If the optic chiasm is not affected, conservative management with pituitary function tests every 6–12 months and tumor size monitoring with annual MRI for 5 years may be decided upon. If the tumor remains far away from the chiasm and its does not increase during monitoring, the radiographic study may be repeated every 2 years.
SurgeryWhen clinical signs of compression are found, surgery is the treatment of choice. Age should not be considered by itself a contraindication for surgery.23 The transsphenoidal approach is the most commonly used today, while the endoscopic procedure allows for a better view of difficult to access areas such as the suprasellar and parasellar areas and the medial wall of the cavernous sinus. Cure is less likely in large tumors, when they have a lobulated suprasellar shape, or if marked cavernous sinus invasion exists.31 Roelfsema et al. analyzed publications of surgical series from the past three decades and reported remission in 44.4% of NFPAs after surgery. This percentage, significantly lower than that found in acromegaly, prolactinomas, and ACTH-secreting pituitary adenomas (60.9%, 61.7%, and 72.7%, respectively), could be due to the greater size of NFPAs at diagnosis. These authors did not find better results in more recent endoscopic series, although there were fewer complications.37 However, DeKlotz et al., in another retrospective meta-analysis of the literature, reported wider tumor resections when endoscopic procedures were used,38 while in a review Swearingen concluded that the endoscopic procedure was superior because it achieved tumor remission in 66–93% of patients with NFPAs.39
Tumor mass resection resolves headache in almost 100% of patients and improves visual disturbances in 80%.40 Vision recovery may continue up to 1 year after surgery.41 The prevalence of anterior hypopituitarism after surgery ranges from 30% to 70%. Pituitary function recovery is less common than in functioning adenomas,42 but has increased with the introduction of endoscopic procedures. Messerer et al. found improvements in hormone deficiencies in 56% of patients with NFPAs treated with endoscopy and in only 25% of those undergoing standard microscopic sublabial transsphenoidal surgery (p=0.01).43 The frequency of new hormone deficiencies is similar to that seen after surgery for prolactinoma or acromegaly and significantly lower than that found in ACTH-secreting adenomas.37
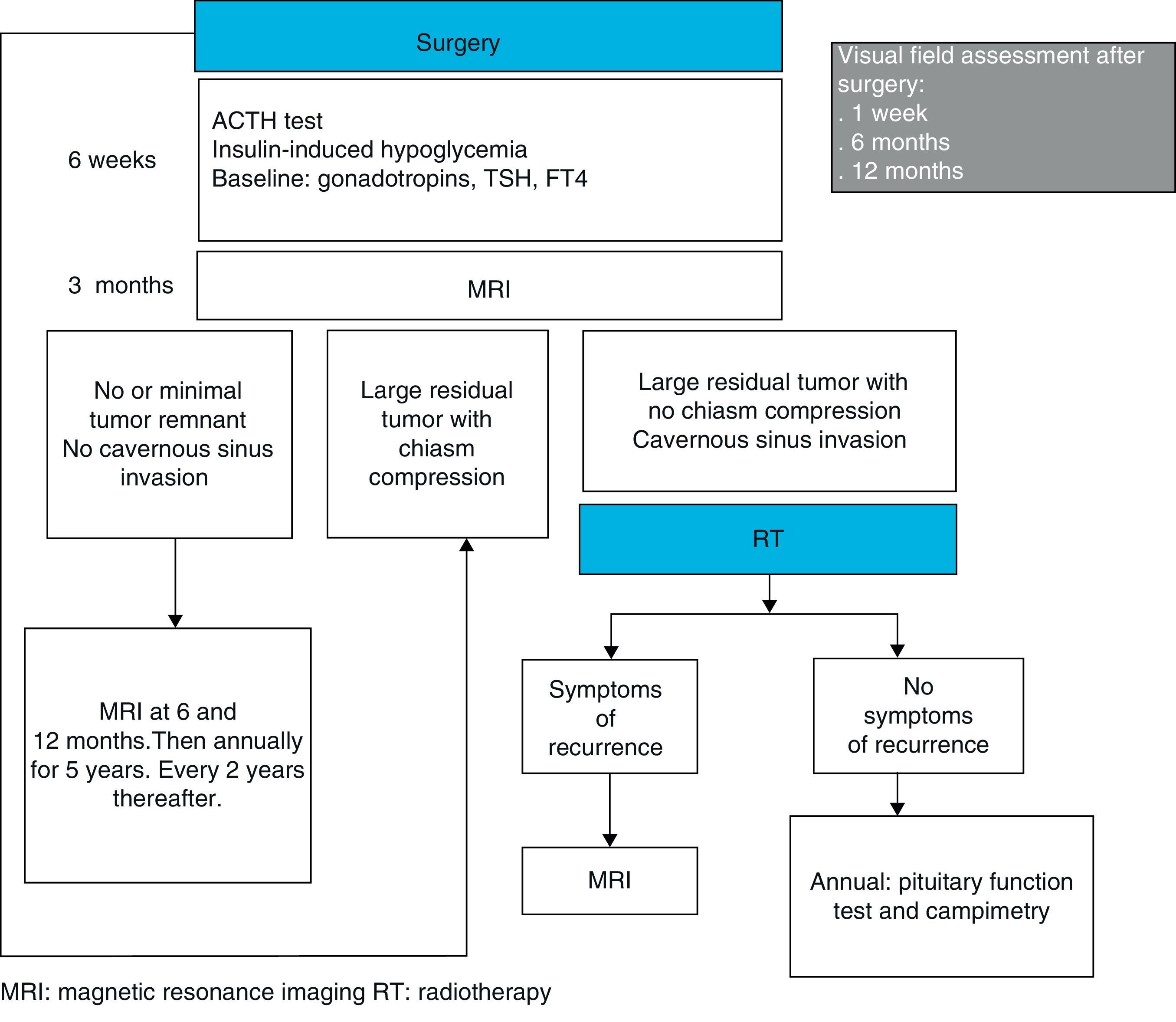
Patient follow-up after surgery may be performed, as suggested by Wass et al., by assessing pituitary function six weeks after surgery, by checking for persistent residual tumor with MRI at 3 months, and by examining visual fields at one week, 6 months, and 1 year. If tumor resection is complete, radiographic monitoring with MRI may be repeated every 6 months in the first year, every year for the next 5 years, and every 2 years thereafter44 (Fig. 1).
RadiotherapyStandard fractionated radiotherapy (RT) is reserved for inoperable tumors where radiosurgery cannot be used due to size, poor tumor mass definition, location close to the chiasm, or tumor extension. Although RT may achieve control in 93% of patients and prevents tumor progression in 75–90% of patients at 20 years, it is not free from undesirable side effects such as the occurrence of new hormone deficiencies or visual damage. There are other complications, traditionally attributed to RT, but increasingly questioned in recent years. Brummelman et al. showed greater cognitive impairment in patients with treated non-functioning macroadenomas as compared to the general population, but did not find any differences between those treated with RT and those undergoing surgery alone.45 Sattler et al. found that patients with pituitary adenomas treated with RT after surgery have an incidence of second tumors similar to patients treated with surgery alone. The authors also reported increased mortality in patients with pituitary adenomas as compared to the general population, although no differences were seen between patients treated and not treated with RT.46 Hypopituitarism and high glucocorticoid replacement doses47 may contribute to this increased mortality seen in NFPAs.
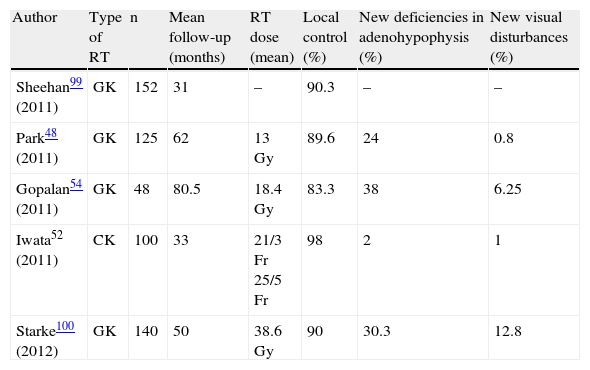
In recent years, radiosurgery procedures have been used in NFPAs with promising results (Table 1). Radiosurgery may be used as the first treatment option in some selected patients with high surgical risk and achieves tumor control in 48.1% of cases.48 However, radiosurgery prevents adequate histological diagnosis in a type of tumor that has no biomarkers. When used as the second-line treatment, radiosurgery achieves tumor control in 83–97% of patients and tumor regression in 42–78%. Kim et al., reviewed the literature on the various radiosurgery procedures used for the treatment of pituitary adenomas, including NFPAs, and concluded that they are all effective and safe, minimally invasive therapeutic options which are useful for tumors <4cm3 at least 3mm away from the optic chiasm and refractory to other treatments. The authors also suggested that doses ranging from 18 to 20Gy are the most adequate for NFPAs.49
Treatment of non-functioning pituitary adenomas with stereotactic radiotherapy procedures.
| Author | Type of RT | n | Mean follow-up (months) | RT dose (mean) | Local control (%) | New deficiencies in adenohypophysis (%) | New visual disturbances (%) |
| Sheehan99 (2011) | GK | 152 | 31 | – | 90.3 | – | – |
| Park48 (2011) | GK | 125 | 62 | 13Gy | 89.6 | 24 | 0.8 |
| Gopalan54 (2011) | GK | 48 | 80.5 | 18.4Gy | 83.3 | 38 | 6.25 |
| Iwata52 (2011) | CK | 100 | 33 | 21/3Fr 25/5Fr | 98 | 2 | 1 |
| Starke100 (2012) | GK | 140 | 50 | 38.6Gy | 90 | 30.3 | 12.8 |
CK: cyberknife; Fr: fractionated; GK: gamma knife; Gy: gray; RT: radiotherapy.
The occurrence of new hormone deficiencies in adenohypophysis has been reported to be correlated to high radiation doses.50,51 Iwata et al., using hypofractionated stereotactic RT (21Gy in three fractions or 25Gy in five fractions), detected the occurrence of new hormone deficiencies in only 2% of patients.52 The results of this procedure are promising, but longer patient follow-up is needed to ascertain its true value. Marek et al. suggested that if doses lower than 15Gy and 17Gy are administered to the pituitary gland and distal infundibulum respectively, the development of hypopituitarism may be prevented in patients treated with a gamma knife.53 However, Gopalan et al. also assessed the effects of gamma knife radiosurgery in 48 patients with NFPAs and found no dose per volume of functioning pituitary tissue below which there was no risk of hypopituitarism, possibly because during RT the normal pituitary gland always receives some radiation.54 The concomitant use with RT of mTOR inhibitors (rapamycin and its derivatives) may allow for the use of lower radiation doses, while achieving adequate therapeutic responses. Theoretically, this would increase the chance of preserving pitutary function, but it is still too early to recommend this association because reported studies55 are few, include few patients, and no prospective studies are available.
Drug treatmentIn NFPAs, dopamine agonists and somatostatin analogues may achieve modest tumor reductions in only a small number of patients. They are therefore not considered as first-choice treatments.56,57
NFPAs have D4 dopamine receptors in 17% of patients58 and D2 receptors in 21.6%.59 Gonadotropinomas and null cell adenomas are the NFPA subtypes with a relatively greater expression of D2 receptors.60 The expression of D2 receptors is very low in silent corticotroph adenomas and plurihormonal tumors.60 This receptor distribution suggests that dopamine agonists may be effective in only a small proportion of NFPAs. However, it has been reported that they may be able to stabilize visual disturbances in 70% of patients and tumor volume in more than 60% of patients.52 Few studies are available on the value of dopamine agonists after surgery,61 but the use of cabergoline could be considered in patients with surgical remnants, particularly in silent adenomas with immunohistochemistry positive for PRL.
The expression of somatostatin receptors (SSTRs) in pituitary adenomas is highly variable. Studies conducted to test SSTRs in NFPAs were not conclusive: while some found SSTR3 to be the dominant receptor subtype expressed by these tumors,62 others found a greater and more prevalent expression of SSTR2 mRNA.63 Even the same working groups have produced several reports with conflicting results,64,65 possibly due to the use of different antibodies. Ramírez et al. used specific monoclonal antibodies in NFPAs and found the expression of SSTR2, SSTR3, and SSTR5 receptors, of which the latter was the most commonly found.66 This may have therapeutic implications for the future, and the authors themselves suggested the possibility of using some somatostatin analogues for the treatment of NFPAs, mainly pasireotide, which is able to act upon SSTR3. With octreotide, tumor size reduction has been seen in 5% of patients, and disease stabilization in 83%.54
The treatment of tumor remnant after surgeryTotal tumor resection is a significant predictor of long-term cure,67 and some studies,25,41 but not all,68 found that tumor remnant is an adverse marker of subsequent growth. The discrepancies are probably partly due to different follow-up times. The risk of recurrence also appears to be related to age, and is higher in patients under 60 years of age.69
If tumor remnant persists after surgery, lower recurrence rates have been reported with the use of standard RT in the early postoperative period as compared to the use of RT only if remnant tumor growth is shown.70 For this reason, RT should be used whenever there is no certainty of complete tumor resection. However, tumor remnant growth is uncommon (9.7–13.5%) in both retrospective67 and observational studies.68 The authors therefore suggest that RT should only be used if tumor remnant growth is seen to prevent potential unnecessary sequelae for patients.67 On the other hand, a delay in the indication of RT does not worsen prognosis, because patients treated with standard RT once tumor remnant growth is detected also show stabilization or regression of pituitary adenoma.70
As the balance between the benefits and the potential undesirable effects of postoperative irradiation is not known, treatment decision should be individualized. In general, if the tumor remnant is small, its size may be monitored with MRI every 6 months in the first year, annually for the next 5 years, and every 2 years thereafter.44 If the remnants are big, are located close to the optic chiasm or compress it, repeat surgery may be performed 4 months after the first procedure.23 If initial surgery consisted of the standard microscopic transsphenoidal procedure, the endoscopic procedure may be considered to try and improve the results.71 If the tumor remnant occupies the cavernous sinuses or is >1cm in size but does not cause optic nerve compression or is not located close to structures that may be affected by their growth, RT may be used72 (Fig. 1). Because of the low growth rate of most NFPAs, some authors only recommend RT if the life expectancy of the patient is longer than 10 years.
Wass et al., also suggested that, after RT, patients should be annually monitored with pituitary function tests and campimetry. In addition, they suggested that radiographic monitoring with MRI should only be performed if symptoms or signs leading to a suspicion of new tumor growth occur44 (Fig. 1).
The treatment of tumor recurrence after surgeryAlmost 30% of patients (10–69%) experience tumor recurrence within 5–10 years of surgery. The microscopic invasion of the dura mater that may occur in up to 94% of macroadenomas with suprasellar extension at the time of surgery does not appear to be a predictor of recurrence. In the meta-analysis conducted by Roelfsema et al., with a mean follow-up of 5.13 years, tumor recurrence was reported after surgery in 10.57% of NFPAs. This recurrence rate is lower than that found in prolactinoma, similar to that reported in ACTH-secreting adenoma, and significantly higher than the rate seen in acromegaly. Although most NFPA recurrences occur in the first 5 years after surgery, a non-negligible number occur in the next 5 years, and follow-up of these patients should therefore be prolonged for at least 10 years. There is no agreement in the analyzed studies as to whether age, sex, tumor size, tumor invasion, or histology should be considered predictors of recurrence.37
When tumor recurrence occurs, repeat surgery may be performed, but this involves greater risks than initial surgery and has less chance of success. If the tumor has grown toward the cavernous sinuses or other brain structures that prevent total resection, or surgery cannot be performed, RT may be indicated.
Aggressive non-functioning pituitary adenomasNot all NFPAs behave similarly. Although they usually grow slowly, tumors are sometimes aggressive and show continuous growth despite the treatments used, including surgery and RT. The term carcinoma should only be reserved for tumors showing craniospinal or systemic metastases.73
According to the 2004 classification of the World Health Organization, “atypical pituitary adenoma” is a potentially aggressive tumor showing increased mitotic index, invasive growth, a Ki67 index value >3%, and nuclear p53 positivity usually in more than 5% of cells. The frequency of this type of adenoma ranges from 2.7%, as reported in the German register of pituitary tumors, to 15% according to Zada et al.,74 but the exact prevalence of aggressive NFPAs is unknown. Aggressive NFPAs may clinically be suspected based on rapid growth after surgery and large initial size, with local invasion in 35–40% of cases. The predictive value of the Ki67 index is highly controversial, particularly in NFPAs.75 Some, but not all, studies29 found a relationship between the Ki67 index and the recurrence rate of NFPAs. There is no agreement either on the Ki67 index value which best predicts tumor aggressiveness. The value of 3% proposed by the World Health Organization has low sensitivity (53.8%) and high specificity (89.5%).76 It is probably better to consider the different types of pituitary adenoma and to determine different maximum Ki67 levels for each of them, as has been proposed by several authors.76,77 The relationship of p53 with the aggressiveness of NFPAs is not clear either.29
Few studies are available on the molecular factors related to the aggressivenes of sporadic NFPAs, and it has been suggested that the clonal origin of recurrent tumors may be different from that of initial tumors. The comparison of normal pituitary glands with tissue from pituitary adenomas provided evidence which suggested that the aggressiveness of these adenomas is related to the presence of the N-terminal truncated isoform of fibroblast growth factor receptor 4 (FGFR4) and to the overexpression of the polysialylated neural cell adhesion molecule78 and various metalloproteases.79 A genetic and molecular study conducted by Galland et al. compared 22 invasive NFPAs to 18 non-invasive NFPAs. Of the 35 genes studied, only MYO5A and IGFBP5 were expressed in different form in the two subgroups. Immunohistochemistry showed greater positivity in MYO5A expression in invasive tumors, and this may be a helpful marker of aggressiveness.80 The invasiveness of some adenomas has also been associated with the overexpression of PTTG,81 but it is not known whether this is related to greater tumor aggressiveness. While some studies found protein PTTG1 to positively correlate to the Ki67 index82 and attributed to it the ability to predict tumor recurrence with 79.2% sensitivity and 100% specificity,83 others found no relevant association with proliferation indices, size, or tumor invasiveness.
It is also thought that microvessel density in angiogenesis may be a tumor invasiveness factor, regardless of cell proliferation. Angiogenic factors, such as endocan and cyclooxygenase-2 (COX-2), could be markers predicting recurrence. Endocan, a proteoglycan secreted by endothelial cells, is related to the aggressiveness of various tumors. Endocan has recently been detected in tissue from pituitary adenomas. and its immunoreactivity in relation to tumor progression and size, increased mitosis, and p53 expression has been shown.84 COX-2 is expressed in 83–100% of pituitary adenomas, and although some authors have reported greater COX-2 overexpression in NFPAs as compared to all other adenomas, other authors think that this is not a predictor of tumor recurrence or progression.85
There are clinical studies suggesting that the imbalance between the actions of estrogen receptors α and β is associated with the invasion of tumors of epithelial origin. Both estrogen receptors have been detected in pituitary tissue, and invasive NFPAs have been shown to have higher levels of estrogen receptors α (p<0.01) and lower levels of estrogen receptors β as compared to non-invasive NFPAs.86 This imbalance may contribute to the aggressive behavior of some NFPAs.
The greater aggressiveness of silent corticotroph adenomas as compared to all other NFPAs has begun to be questioned. Alahmadi et al. found no greater tumor recurrence in 20 patients with silent corticotroph adenomas.87 In a retrospective study, Ioachimescu et al. compared the clinical, radiographic, and hormonal characteristics of 33 silent corticotroph macroadenomas to those of 126 non-functioning macroadenomas. Patients with silent corticotroph adenomas were younger and had more hormone deficiencies and a greater trend to cavernous sinus invasion, but postoperative tumor growth was similar in both groups using a similar adjuvant RT protocol in cases with tumor remnant. According to the authors, these findings suggest that the management plan of NFPAs should probably not be modified based only on a positive result for ACTH in immunohistochemical analysis. However, they also recommend that close patient follow-up should be continued.88 Other authors think that although recurrence is not more common in silent corticotroph adenomas, if recurrence does occur, it is more aggressive than in any other NFPA.35
The treatment of aggressive non-functioning pituitary tumorsFew therapeutic options are currently available for aggressive NFPAs. Repeat surgery may be considered if the tumor is close to or compresses the chiasm, but surgery may not be curative even when it controls the symptoms for variable time periods.89 RT, particularly if used early, may prevent tumor growth, but only rarely induces tumor regression. On the other hand, most aggressive tumors show resistance to drug treatment with dopamine agonists or somatostatin analogues.
Several chemotherapeutic treatments have been used, but the response achieved is almost always partial and transient.90 Experimentally, everolimus, an mTOR pathway inhibitor, decreases cell viability by inducing apoptosis, and concurrent treatment with pasireotide, but not with cabergoline, has an additive effect.91 Temozolomida (TMZ), a second-generation oral alkylating agent, was first used for the treatment of pituitary carcinomas,92 showing significant activity against this type of tumor. Because of this, it was subsequently used in patients with aggressive pituitary adenomas. However, aggressive NFPAs and non-functioning carcinomas show the worst response to treatment with TMZ, with tumor control achieved in only 38–40% of patients,93,94 as compared to 73% of prolactinomas and 60% of ACTH-secreting tumors. Among NFPAs, gonadotropinomas show a better response rate (66.6%) than null cell tumors (20%).93
The cytotoxic effect of TMZ is based on DNA methylation at the O6 position of guanine. TMZ is administered orally, activates at physiological pH, and shows little between-patient pharmacokinetic variation.95 TMZ is well tolerated as compared to other chemotherapeutic agents. Several dosing schemes have been proposed (Table 2) and although the most efficacious for pituitary tumors is not yet known, the most commonly used is the standard scheme (200mg/m2 for 7 days every 28 days). The maximum length of treatment has not been established either. A lack of response to TMZ after three cycles predicts for TMZ resistance. On the other hand, an initial response does not always ensure mid- or long-term control.93
Temozolomide dosing schemes.
| Standard scheme | 150–200mg/m2/day for 5 days every 28 daysOccasionally:- Dose of initial cycle: 150mg/m2/day for 5 days- Subsequent doses, given good tolerability: 200mg/m2/day for 5 days every 28 days |
| Less common schemes | 50–75mg/m2/day continuously150mg/m2/day on days 1–7 and 14–21 in a 28-day cycle80–100mg/m2/day for 21 days in 28-day cycles |
The presence of O6-methylguanine-DNA-methyltransferase (MGMT), a DNA repair enzyme, inside tumor cells may induce resistance to TMZ action and poor therapeutic response to the drug. It has therefore been suggested that MGMT expression should be assessed by immunohistochemistry before TMZ is administered to aggressive adenomas.96 There are, however, few studies available quantifying MGMT expression. Moreover, it is not yet known what precisely constitute high, moderate, or low levels. Widhalm et al. showed low MGMT expression in 50% of non-functioning tumors with aggressive behavior.97 Using a semiquantitative analysis of MGMT expression, Raverot et al. found that the value of this measurement to predict the response to treatment with TMZ was only 25% for patients with NFPAs, clearly lower than the values found in prolactinomas (67%), ACTH-dependent Cushing (79%), and acromegaly (100%). The authors therefore concluded that MGMT expression should not be determinant in the selection of patients who might benefit from treatment with TMZ.93
The most common toxicity of TMZ is myelosuppression, with an occurrence of thrombocytopenia in 7%-17% of cases and less frequently, neutropenia.98 The peak incidence of these adverse effects occurs between days 21 and 28 of each administration cycle, with recovery in 1–2 weeks. There is no evidence of cumulative hematological toxicity. The risk of myelotoxicity appears to be greater when high, continued TMZ doses are used and when MGMT expression before treatment is low. The occurrence of myelodiyplastic syndrome, aplastic anemia, or hematological cancer is less common. Other non-hematological undesirable effects include nausea and vomiting (34%), asthenia (20%), skin rash, headache, anorexia, and diarrhea. When high, continuous TMZ doses are used or concomitant RT or corticosteroids are administered, the risk of infection is increased, and the concurrent administration of trimethopim-sulfametoxazole or pentamidine is therefore recommended in these situations.
Conflicts of interestThe author states that she has no conflicts of interest.
Please cite this article as: Cámara Gómez R. Tumores hipofisarios no funcionantes: actualización 2012. Endocrinol Nutr. 2014;61:160–170.