Prevention of high incidence of cardiovascular disease in diabetes is one of the challenges of endocrinology. Validation of new biomarkers that may contribute to a better assessment of cardiovascular risk and help implement treatment strategies is one of the promising approaches in research on prevention and reduction of cardiovascular risk. Modification of low density lipoprotein (LDL) is a key element in development of atherosclerotic lesions. Several pathophysiological characteristics of diabetes are crucial for the LDL of these patients to have higher modification rates as compared to the healthy population. Diabetic dyslipidemia, hyperglycemia, and oxidative stress synergistically promote the occurrence of lipoperoxidation, glycosylation and glycoxidation processes, which will generate modified lipoproteins that stimulate development of atherosclerosis. This article reviews the role of different types of modified LDL in development of atherosclerosis in diabetes, as well as the possibility of using its quantification in cardiovascular risk prediction.
La prevención de la alta incidencia de enfermedad cardiovascular en la diabetes es uno de los retos de la endocrinología. La validación de nuevos biomarcadores que puedan contribuir a una mejor evaluación del riesgo cardiovascular y ayuden a implementar estrategias terapéuticas es una de las aproximaciones prometedoras en la investigación dirigida a la prevención y a la reducción del riesgo cardiovascular. La modificación de las lipoproteínas de baja densidad (LDL) es un elemento clave en el desarrollo de la lesión arteriosclerótica. Varias características fisiopatológicas de la diabetes contribuyen decisivamente a que la LDL de estos pacientes tenga unos índices de modificación más elevados que la de la población sana. La dislipidemia diabética, la hiperglicemia y el estrés oxidativo favorecen de manera sinérgica la aparición de procesos de lipoperoxidación, glicosilación y glicoxidación que van a generar lipoproteínas modificadas que estimulan el desarrollo de la arteriosclerosis. Este artículo revisa el papel de los diferentes tipos de LDL modificada en el desarrollo de la arteriosclerosis en la diabetes, así como en la posibilidad de utilizar su cuantificación en la predicción del riesgo cardiovascular.
Cardiovascular disease derived from atherosclerotic conditions is the leading cause of death in patients with diabetes mellitus. This disease has a number of characteristics contributing to an increased cardiovascular risk (CVR) through independent mechanisms. Overall, three events with a relevant role in the development of atherosclerosis may be distinguished in patients with diabetes: (1) diabetic dyslipidemia; (2) non-enzymatic glycosylation of proteins; and (3) oxidative stress.1,2 These processes are in principle independent but, as we will attempt to explain in this review, are closely interconnected events (Fig. 1). The consequence of the high incidence of these conditions in diabetes is that lipoproteins, mainly low density lipoproteins (LDL), of these patients are modified, so that they lose their natural characteristics and function.3 These modified LDLs are a determinant for the accelerated development of atherosclerosis experienced by subjects with diabetes. The direct implication of modified LDL in the evolution of atheromatous lesions suggests that quantification of LDL in plasma could be a very helpful tool for both the prediction of CVR and the monitoring of treatment aimed at decreasing this risk.4
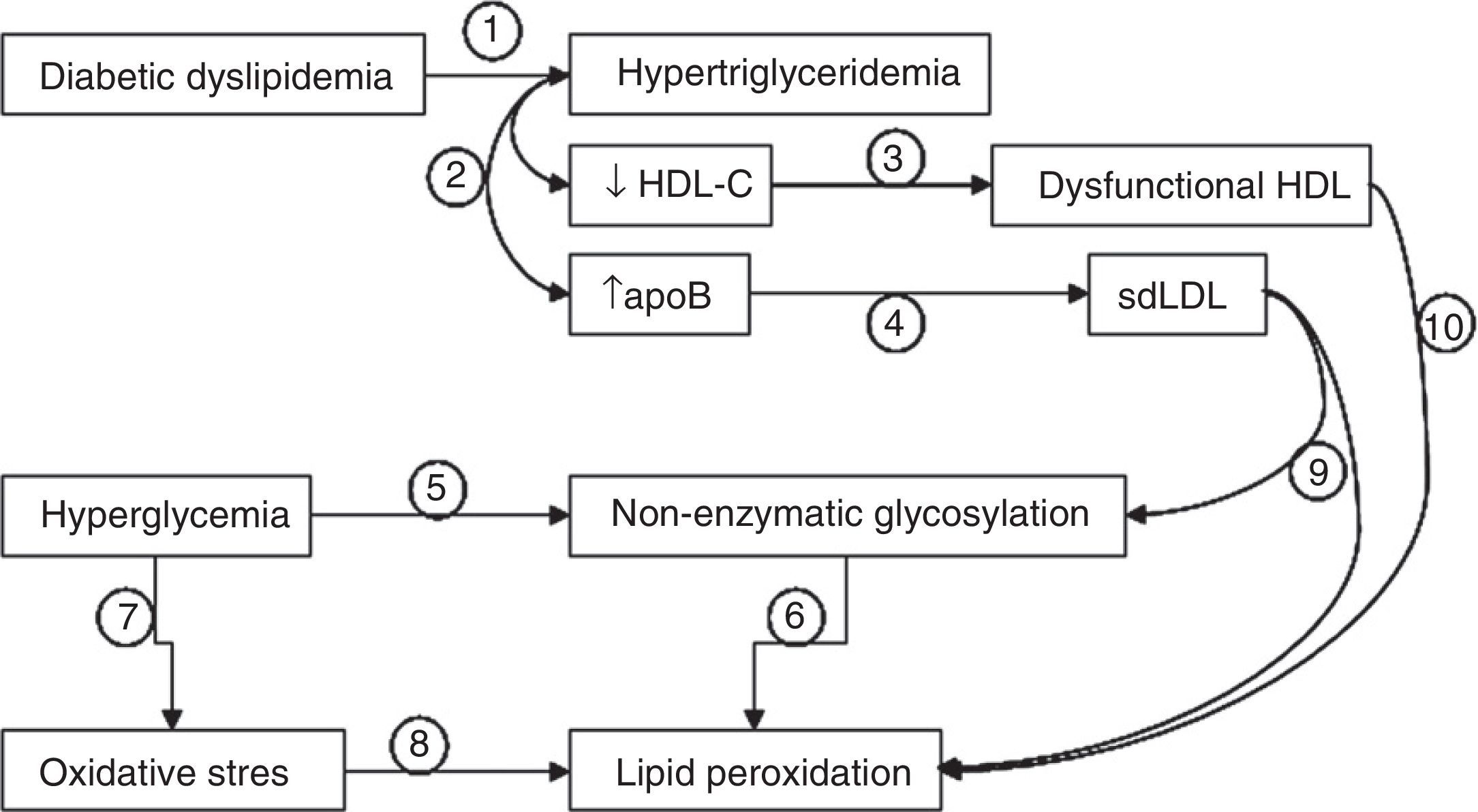
Interactions between diabetic dyslipidemia, hyperglycemia, and oxidative stress. Diabetic dyslipidemia is characterized by hypertriglyceridemia (1), which results in decreased HDL cholesterol levels and increased apoB levels (2). In these patients, HDL is not only quantitatively decreased, but is partly dysfunctional (3), and elevated apoB levels are associated with the presence of small, dense LDL particles (sdLDL). On the other hand, hyperglycemia promotes the non-enzymatic glycosylation of proteins (5), a process that stimulates the occurrence of lipid peroxidation events (6). Hyperglycemia also increases cellular oxidative stress (7), enhancing lipid peroxidation (8). The fact that LDL particles are small and dense (9) (more oxidizable and glycosylable) and the fact that HDL is partly dysfunctional (10) (less antioxidant potential) also contribute decisively to increases in non-enzymatic glycosylation and lipid peroxidation.
Metabolic syndrome (MS), defined as abdominal obesity, insulin resistance, hypertension, and an abnormal lipid profile,5 is frequently associated with type 2 diabetes. Although all four factors are associated with the early development of atherosclerosis, an abnormal lipid profile is probably the factor most directly related to atherogenesis. This lipid profile, known as diabetic or atherogenic dyslipidemia, is characterized by hypertriglyceridemia, decreased high density lipoprotein (HDL) cholesterol levels, increased apolipoprotein B (apoB) levels,6 and increased postprandial lipidemia.7,8
ApoB is the main protein component of atherogenic lipoproteins, LDL and very low density lipoproteins (VLDL). However, LDL cholesterol levels are usually normal. Since 80–90% of apoB is associated with LDL, this observation may seem paradoxical. This is explained by the abundance of small, dense LDL (sdLDL) particles with lower relative cholesterol content and greater apoB content, resulting from the deficient metabolization of VLDL.9 Thus, although patients with diabetes often have normal plasma total cholesterol levels, their lipid profile is far from what may be considered as an atheroprotective factor. This suggests that, despite the fact that drug treatment of dyslipidemia is quantitatively efficient,10 the incidence of cardiovascular events in the diabetic population continues to be high.
Significant qualitative changes in HDL and LDL occur in diabetic dyslipidemia. The effect on the antiatherogenic role of HDL, which is determinant for reverse cholesterol transport and has anti-inflammatory and antioxidant properties protecting LDL from oxidation, should first be considered.11 HDL levels are not only decreased in patients with diabetes, but are also partially dysfunctional, with a decreased capacity to stimulate reverse cholesterol transport and a decreased antioxidant and anti-inflammatory capacity.12 HDL dysfunctionality therefore promotes the formation of oxidized LDL (oxLDL).
sdLDL is more atherogenic than LDL of normal size and density because of a number of distinctive characteristics. It has less affinity for the LDL receptor, which implies a lower plasma clearance rate and a longer residence time in circulation. In addition, sdLDL crosses the endothelial barrier more easily than native LDL, because this process mainly depends on lipoprotein particle size. It also binds with greater affinity to proteoglycans forming the arterial wall, promoting subendothelial lipoprotein retention. Moreover, sdLDL has greater susceptibility to modification by oxidative and non-enzymatic glycosylation mechanisms.13 The latter relates diabetic dyslipidemia to the other two processes involved in the abovementioned increase in CVR, namely non-enzymatic glycosylation and oxidative stress.
Non-enzymatic glycosylationA continued hyperglycemia state enhances the non-enzymatic glycosylation processes of the different macromolecules. This has a highly significant impact on protein function. This process occurs when glucose reacts with amino acids containing amino groups (mainly lysine and arginine) to form a Schiff base and, subsequently, a stable product called the Amadori product.14 This modification may affect the function of a very high number of proteins. Although the proteins most affected by non-enzymatic glycosylation are of course structural proteins with a long half-life, lipoproteins may also be glycosylated during their residence time in circulation.15 However, the most widely accepted concept is that this process is enhanced in LDL retained in the arterial wall for a longer time. This modification affects LDL function, as exemplified by the fact that glycosylated LDL loses affinity for the LDL receptor.16 SdLDL, common in patients with diabetes, is more susceptible to non-enzymatic glycosylation processes than LDL of normal size,17 which confers on this modification a greater relevance in patients with diabetic dyslipidemia. Non-enzymatic glycosylation in turn induces the formation of oxygen free radicals with the resultant stimulation of the oxidative processes, a phenomenon known as glycoxidation.18 This process results in a rearrangement of the molecular bonds and leads to the formation of advanced glycation end-products (AGE).18 This is a heterogeneous group of compounds which irreversibly alter protein function. All these processes occur in vivo, which has allowed for the detection of glycosylated LDL (glLDL) and modified LDL with AGE (AGE-LDL) in plasma circulation.19 AGE-LDL, both generated in vitro and isolated from plasma circulation, has inflammatory properties and induces apoptosis in vascular wall cells,20–22 processes which are both involved in the development of atherosclerosis.
The relatively short half-life of LDL in circulation (2.5–3.5 days) has always been an argument against a significant in vivo effect of non-enzymatic glycosylation on LDL during plasma circulation because, in the absence of reducing agents, 6–7 days are usually required for glucose to significantly change proteins.23 Thus, it has implicitly been assumed that the formation of glLDL, and particularly of AGE-LDL, mainly occurs in LDL which has been retained in the arterial wall for a longer time than its plasma life.23 It is therefore assumed that the glLDL and AGE-LDL detected in plasma circulation were formed in damaged arterial wall areas and that their presence in blood reflects the development of atherosclerotic areas.
An additional type of change related to hyperglycemia but not directly affecting glucose is modification by methylglyoxal (MG) or other similar compounds.24 Different researchers have studied the effect of dicarbonyl glucose metabolites with a high reducing power, the most important of which is MG. This metabolite is able to rapidly react with arginine residues. Studies by Thornalley et al. showing the presence in blood of LDL modified by MG (MG-LDL) and that its levels were increased in patients with diabetes and decreased after treatment with metformina are particularly important.25 LDL minimally modified by MG has a number of atherogenic characteristics including smaller size, increased susceptibility to aggregation, and greater binding affinity for proteoglycans in the arterial wall.26 Arginine modification with MG results in a heterocyclic compound (hydroimidazolone) which is part of the heterogeneous family of AGE compounds, so that MG-LDL is a specific form of the AGE-LDL group. However, the relevant point to make about MG-LDL, within the whole group of AGE-LDL, is that because of the high reactivity of MG, it could be formed during the plasma circulation of LDL.
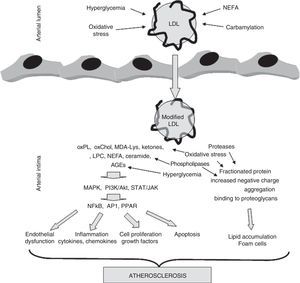
Oxidative stressThe modification of proteins by glycosylation and subsequent AGE formation is not the only mechanism by which oxidative stress is involved in the development of atherosclerosis in patients with diabetes. In fact, increased systemic oxidative stress is a characteristic of diabetes.1 The main cause is probably that, as the result of hyperglycemia, an increase occurs in mitochondrial activity which favors the production of radical oxygen species (ROS).27 Because of this, changes in the parameters quantifying oxidative stress are very frequently detected in plasma from these subjects. Oxidative stress plays a particularly significant role in the subendothelial space of the arterial wall, a microenvironment surrounded by metabolically active cells (endothelial cells, smooth muscle cells, macrophages) which generate ROS and lacking the abundant antioxidant defenses of blood plasma. Oxidative modification may affect all macromolecules, but lipoproteins, and specifically LDL, are highly sensitive to oxidative attack by ROS.28 These radicals mainly oxidize the unsaturated fatty acids of phospholipids located on the lipoprotein surface, a process called lipid peroxidation. Three decades of research, starting with pioneering research by Steinberg et al. in the early 1980s, have shown that oxidative LDL modification is a key event in the development of atherosclerosis.28–30 Unlike native, unmodified LDL, which has no potentially atherogenic properties, oxLDL is able to promote and/or be involved in virtually all events occurring during the evolution of the atherosclerotic lesion. Thus, oxLDL is able to induce a massive intracellular accumulation of cholesterol esters by macrophages, inducing foam cell formation, which is probably the most characteristic pathological feature of the atherosclerotic lesion.31 This is due to a loss of affinity for the LDL receptor, which is associated with an increased affinity for scavenger receptors (SR), whose expression is not regulated by intracellular cholesterol content. In addition, oxLDL may induce different arterial wall cells to express cytokines, chemokines, and growth factors. OxLDL thus promotes the chronic inflammatory process and cell proliferation characteristic of atherosclerosis.30,32,33 It is also cytotoxic and apoptotic, promoting the formation of the necrotic core found in advanced atheromatous lesions.34Fig. 2 shows the impact of modification, either by oxidative or other different mechanisms, on LDL and its implication in the development of atherosclerosis.
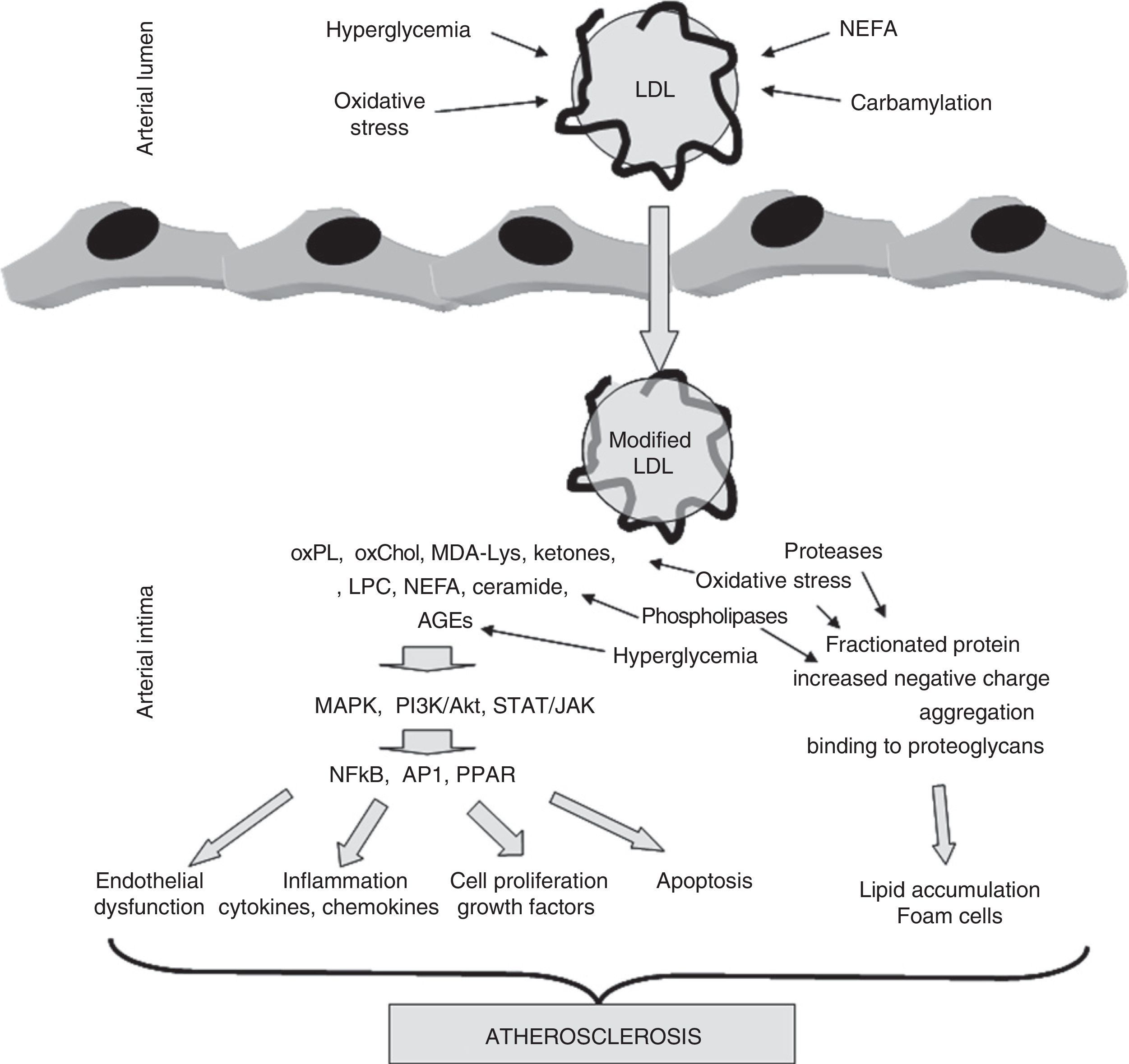
The role of LDL modification in the development of atherosclerosis. LDL may be modified by different mechanisms during its residence time in circulation (hyperglycemia, oxidative stress, carbamylation, NEFA overload) or once it has been trapped into the arterial wall (hyperglycemia, oxidative stress, lipolysis, proteolysis, aggregation). These modifications result in compounds, mainly of a lipid nature (oxidized phospholipids, oxidized cholesterol, ketones, lysophosphatidylcholine, NEFA, ceramide), but also derived from apoB (MDA-Lys adducts, AGE), with inflammatory, apoptotic, and proliferative potential. These compounds activate kinase-mediated signaling pathways (MAPK, PI3K/Akt, TAT/JAK) which stimulate in turn the translocation and/or activation of transcription factors (NFkB, AP1, PPARγ). These factors cause endothelial dysfunction and stimulate the secretion of cytokines, chemokines, growth factors, and mediators of apoptosis. On the other hand, other modifications break down apoB, increase negative charge, promote aggregation, and promote binding to proteoglycans forming the arterial wall. This results in the stimulation of subendothelial LDL accumulation and the formation of foam cells characteristic of atheromatous lesions.
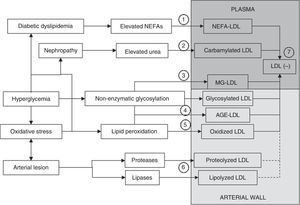
Multiple evidences suggest that LDL may be modified by other mechanisms in addition to oxidative changes and non-enzymatic glycosylation processes. Fig. 3 summarizes the different processes that may generate the different types of modified LDL.35 In atherosclerotic lesions there is an overexpression of lipolytic enzymes such as phospholipase A3 (PLA2), sphingomyelinase (SMase), or cholesterol esterase (CEase), and also of various proteases (metalloproteinases, cathepsins).36–41 LDL retained in the arterial wall is therefore assumed to be affected not only by lipid peroxidation and glycoxidation processes, but also by proteases and lipases. Indirect evidence of this is that lipoproteins isolated from the arterial wall show fragmentation of apoB (representing 98–99% of the protein in LDL) and high contents of various products of the enzymatic breakdown of lipids such as lysophosphatidylcholine, ceramide, and non-esterified cholesterol.31,42,43 Some of these products may also be generated from oxidative processes (apoB fragmentation, lysophosphatidylcholine), but others cannot be explained by these events. In addition, the fact that oxidation products are not excessively elevated in atherosclerotic lesions has resulted in the contribution of oxidative modification to the development of atherosclerotic lesions being played down.44 The lack of positive results in large clinical trials using different antioxidant molecules has also contributed to this.45,46 The current general perception is that, while it is still accepted that lipid peroxidation plays a significant role in atherogenesis, other mechanisms of LDL modification, such as enzymatic modification through lipases or proteases, may play a role even more preponderant than oxidation in the generation of modified LDL in the arterial wall.44,47
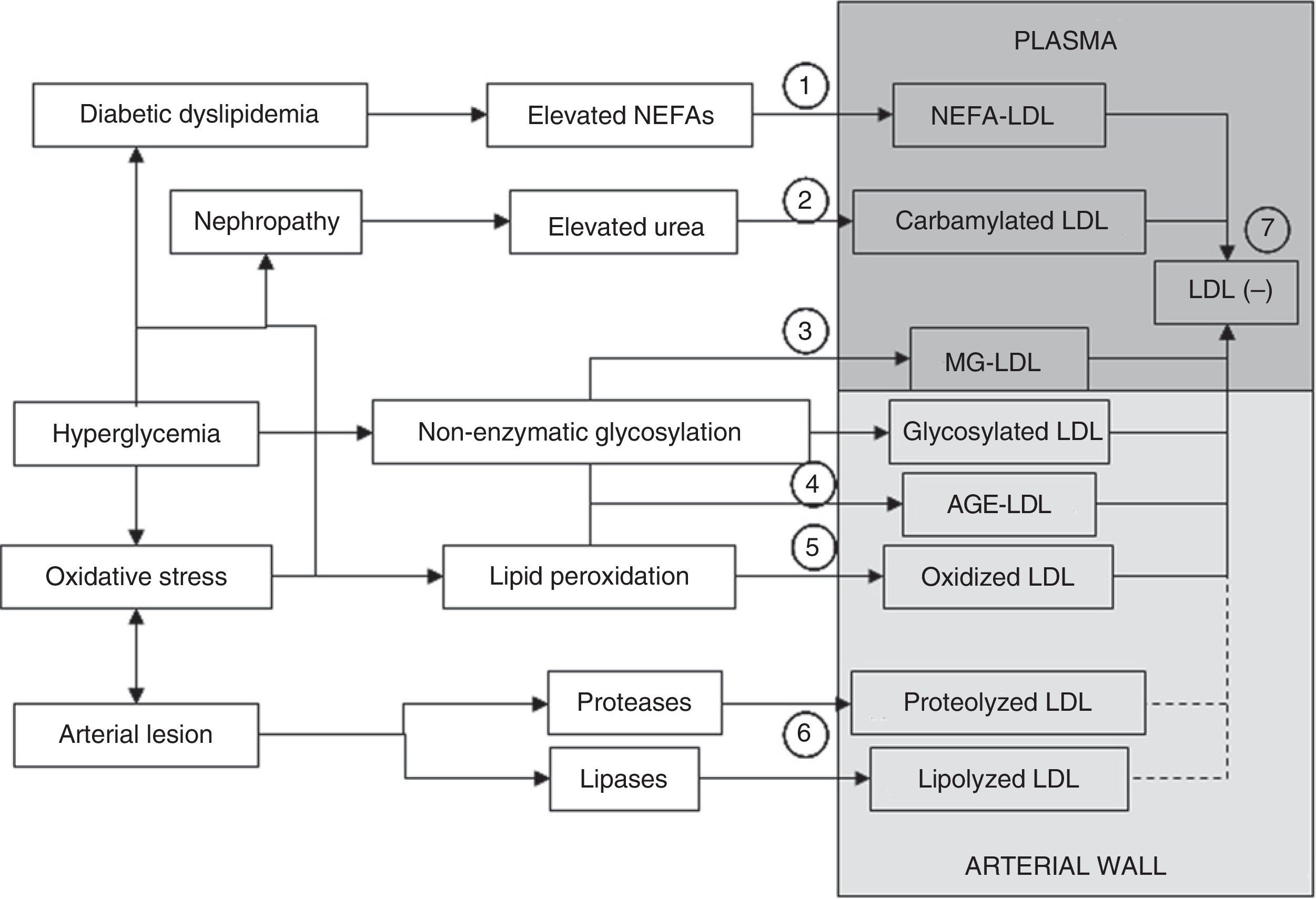
Mechanisms through which modified LDL is formed. LDL may be modified in plasma or the arterial wall by different mechanisms. Elevated plasma NEFA levels characteristic of diabetic dyslipidemia promote LDL overload with NEFAs (1). If nephropathy and hyperuremia exist, LDL carbamylation is stimulated (2). Another process that may occur in plasma circulation is modification by methylglyoxal (MG) (3). Significant modification of LDL in plasma by non-enzymatic glycosylation is more difficult. The formation of glycosylated LDL and AGE-LDL is therefore more likely to occur in the arterial wall (4). Similarly, although oxidative modification may occur in plasma, it is also more likely to occur more extensively in the arterial wall (5). Modification mediated by lipases and proteases necessarily also preferentially occurs in the arterial wall (6). A common characteristic to all these modifications is an increase in the negative electric charge of the LDL particle, which is reflected in the formation of electronegative LDL (LDL(−)), a modified form of LDL that may be isolated from plasma circulation (7). LDL(−) consists at least of NEFA-LDL, carbamylated LDL, MG-LDL, glycosylated LDL, AGE-LDL, and oxidized, and maybe also lipolyzed and/or proteolyzed LDL from the arterial wall.
In addition to the processes described so far, preferentially occurring in the arterial wall, LDL may also be modified by other mechanisms in the bloodstream. The presence of carbamylated LDL in plasma has recently been reported.48,49 Carbamylation is a chemical change generated by a reaction with the cyanate molecule derived from thiocyanate formed from urea.48 This type of modification has been shown to be particularly important in smokers because tobacco smoke promotes the formation of thiocyanate, and in patients with chronic uremia due to severe renal failure. This suggests the possibility that carbamylated LDL is increased in patients with diabetes and kidney disease. However, this has yet to be experimentally confirmed.
The presence in the circulation of desialized LDL, i.e. LDL with a decreased content in sialic acid, one of the carbohydrates forming the enzymatic glycosylation chains of apoB, has also been reported. Desialized LDL is increased in patients with diabetes and is able to induce foam cell formation. It is therefore potentially atherogenic.50 Desialization has been attributed to oxidation processes, because these promote the loss of sialic acid bound to apoB. Desialized LDL may therefore reflect the presence of oxLDL.51
Another LDL modification, which may quantitatively be highly relevant, is overload with non-esterified fatty acids (NEFAs). These compounds are usually transported in blood associated with albumin. When albumin transport capacity is exceeded due to increased plasma NEFA levels, NEFAs bind to other macromolecules, mainly lipoproteins.52 LDL with increased NEFA contents has a greater inflammatory potential and an altered structure which promotes its aggregation.53–55 This phenomenon is important in diabetes, where plasma NEFA levels are frequently increased. In this context, LDL in diabetic patients has been reported to have high NEFA contents.56 This may explain observations in other studies showing that LDL from these subjects is more inflammatory than LDL from subjects with no diabetes, despite the fact that lipid peroxidation indices are not increased.57,58
A property common to the different forms of modified LDL previously described is an increase in the electric charge of the particle.59 Using this characteristic, Avogaro et al. were the first to isolate from plasma a fraction of modified LDL with an increased negative charge they called electronegative LDL(LDL(−)).60 LDL(−) may be considered as a pool that contains the different modified forms of LDL (Fig. 2) present in blood and may account for approximately 5% of total LDL in healthy subjects. LDL(−) is increased 2- to 4-fold in different groups of subjects with high CVR or advanced atherosclerosis, including patients with type 1 and type 2 diabetes.59,61,62 The proportion of LDL(−) is much higher than the values usually reported for oxLDL or AGE-LDL (0.1–1%).63,64 Both oxLDL and AGE-LDL can therefore be considered minority forms within LDL(−) and most LDL(−) is probably LDL with increased NEFA contents, other associated minority apolipoproteins (other than apoB) and/or greater density (sdLDL). LDL(−) therefore reflects the metabolic abnormalities occurring in the different diseases, while oxLDL or AGE-LDL are related to the presence of underlying atherosclerotic lesions.
The value of modified low density lipoprotein as a biomarkerRegardless of the relative significance of each type of modification for the generation of modified LDL and the atherogenic properties conferred to LDL by each of these mechanisms, LDL modification is widely accepted as playing a key role in atherogenesis. Many researchers have therefore suggested that the quantitation of modified LDL may be used as a CVR marker, and may even serve to estimate the extent and evolution of atherosclerotic lesions found in patients with atherosclerosis.4,64 Although the possibility that part of oxLDL and AGE-LDL has formed during its residence time in plasma circulation cannot be ruled out completely, the general perception is that oxLDL and AGE-LDL have been formed in the arterial wall. The occurrence in plasma of these modified LDL forms may therefore reflect the silent presence of active atherosclerotic lesions, because LDL is oxidized and/or glycoxidized in injured areas of the arterial wall but is not found in healthy areas. OxLDL may thus be considered to be not only a biomarker of atherosclerosis, but also a potential indicator of unstable and/or ruptured atherosclerotic plaques which release part of their contents into circulation.64–69
To show this involvement of modified LDL and to assess its value as a biomarker, rapid, reproducible, and relatively simple methods allowing for quantitation in large groups of patients have been developed. From the early 1990s, advances in the development of immunoassays have made it possible to detect the presence of different modified LDL forms in plasma circulation. Holvoet et al. developed an immunoassay able to detect MDA-LDL, a form of modified LDL resulting from oxidation, and were the first to report an increased concentration in patients with atherosclerosis.70 Since then, at least three methods based on different antibodies (4E6, E06, and DLH3) recognizing different oxidative epitopes generated in oxLDL (adduct MDA-Lys, phosphorylcholine, and oxidized phosphatidylcholine respectively) have been marketed.4,70–72 Cohen et al. developed in 1993 an immunoassay method to quantitate glycosylated LDL73 which was marketed soon afterwards. Methods for detecting AGE-LDL have also been developed74 and, more recently, immunoassays able to detect carbamylated LDL49 and LDL(−) have been implemented. However, these latter methods have not been marketed, which has limited to date the development of multicenter studies that may validate these types of modified LDL as CVR markers. The objective of many researchers has been to use these tools to determine whether, in addition to being a causative agent, modified LDL could be quantitated and serve as a biomarker of atherosclerotic disease, providing additional information to the more traditional risk factors. A vast majority of studies have quantitated oxLDL, but studies assessing AGE-LDL and LDL(−) have become increasingly relevant in recent years.
Association of oxidized low density lipoprotein with cardiovascular riskMany population studies have reported increased oxLDL levels in groups of patients with high CVR, including hypercholesterolemia, hypertriglyceridemia, metabolic syndrome, obesity, diabetes, hypertension, and severe kidney disease.4,64,70,75–77 Increased oxLDL levels have also been reported in plasma from patients with angiographically documented atherosclerosis and have been associated with severity of coronary artery disease.65,78–80 However, although the involvement of oxLDL in the development of atherosclerosis is widely accepted, its value as an independent biomarker of CVR is moderate.81–83 This may be due to different reasons. On the one hand, a strong correlation exists with lipid parameters, particularly total cholesterol and LDL, especially in subjects with dyslipidemia.81 This masks the role of oxLDL as a biomarker and plays down the value of its quantitation. Another factor explaining the lack of conclusive studies is that the different immunoassays using antibodies that recognize different epitopes generated during the oxidative process in LDL are not standardized.4,64 As this process is tremendously complex and results, in a more or less sequential form, in products which occur in some oxidation phases and are degraded in other phases, the different immunoassays may detect different oxidative states of LDL. The complete standardization of these methods is clearly needed in order that the results achieved in the studies conducted can be compared.
The observation that oxLDL levels, regardless of the measurement method used, transiently increase during the acute phase of acute myocardial infarction or stroke, and also after percutaneous transluminal angiography, is more consistent and probably more applicable.84–86 These observations support the concept that circulating oxLDL comes from the injured arterial wall and suggest that the quantitation of oxLDL in blood may be a very helpful tool for increasing our understanding of the vulnerability of atheromatous lesions and for the secondary prevention of cardiovascular events in patients with atherosclerosis.
Modified low density lipoprotein as a cardiovascular risk biomarker in diabetesMost studies conducted in patients with both type 1 and type 2 diabetes have shown increased levels of the different types of modified LDL, including oxLDL, glycosylated LDL, AGE-LDL, and LDL(−).62,73,87–92 Overall, it has been noted that poor glucose control is associated with higher levels of modified LDL and that the optimization of glucose control results in decreases in these levels.62,93,94 Similarly to the findings made in studies conducted in subjects without diabetes, treatment with lipid lowering agents also decreases levels of modified LDL in patients with diabetes, with a clear relationship being found with the effects on lipid profile.90,95,96 Thus, its predictive value as an independent factor of clinical cardiovascular events is not completely clear. In type 2 diabetes, where lipid profile is usually altered, studies have found particularly significant discrepancies.97,98 Some studies conducted in populations with diabetes have reported that oxLDL is a predictor of the occurrence of cardiovascular events, although some authors did not find this independent association when lipid profile abnormalities were considered.99–101 However, in contrast to the weak association of modified LDL with clinical events, other studies found independent associations with other markers of the course of atherosclerosis such as carotid intima-media thickness or diabetic nephropathy.102–106
A significant advance in the use of LDL as a biomarker is the quantitation of the immune complexes formed by antibodies and modified LDL (oxLDL-IC o AGE-LDL-IC), mainly in studies conducted in subjects with type 1 diabetes.107 One of the properties of the different types of modified LDL is their immunogenic capacity.108–113 This property has allowed for the detection in plasma of specific autoantibodies against the different modified LDLs, including oxLDL, AGE-LDL, and LDL(−). Various studies have shown that levels of these autoantibodies are associated with the presence of atherosclerotic disease, although many discrepancies exist in this regard. The current evidence would appear to suggest that IgG antibodies are positively associated with the development of atherosclerosis, while IgM antibodies play an atheroprotective role.114
The most relevant studies in this regard were conducted by the group of Virella and Lopes-Virella, mainly in subjects with type 1 diabetes. Using in vitro studies, these authors showed that LDL-ICs have greater atherogenic potential than modified LDL not bound to antibodies.97,115 This could be due to the fact that, in addition to activating the pathway mediated by scavenger receptors recognizing modified LDL, LDL-ICs also activate in monocytes the pathway of the antibody-specific Fc receptor, enhancing the inflammatory process.20,115,116 It should also be noted that most of the oxLDL and AGE-LDL present in plasma circulate as ICs.20,74,117 This supports the concept that immune complexes with oxLDL or AGE-LDL play in vivo a more relevant role in the development of atherosclerosis than free oxLDL and AGE-LDL.
In agreement with this concept, studies conducted by this group have shown that levels of oxLDL-ICs and AGE-LDL-ICs are strongly associated with carotid intima-media thickness and its progression in type 1 diabetes independently of other risk factors.88 These ICs are also associated with the extent of coronary calcification,118 the risk of developing nephropathy,119 and the progression of retinopathy.120 The significance of these studies lies in the fact that they have been conducted on a large population enrolled in the cohort of the DCCTR/EDIC study, and the consistency of the results is therefore high. However, no studies analyzing the association of LDL-ICs with the incidence of coronary events in patients with type 1 diabetes have been conducted yet. Such an association was however analyzed in type 2 diabetics (the VADT cohort).102 Interestingly, the incidence of clinical events in these patients was not found to be associated with oxLDL-ICs or AGE-LDL-ICs, but with the levels of immune complexes of MDA-LDL, a specific form of oxLDL which was not associated with the progression of atherosclerosis in type 1 diabetes.88 The reasons for these differences are not clear, but they may be attributed to the different evolution of lesions between both types of patients with diabetes. The main disadvantage of this test method is the need for a precipitation step of immune complexes before quantitation using an immunoassay for modified LDL. This increases the technical complexity of the measurement, which is therefore difficult to perform in most clinical laboratories.
ConclusionsPlasma levels of different modified LDLs are increased in patients with diabetes, although this observation has often been strongly associated with the presence of dyslipidemia. However, recent studies assessing levels of modified LDL associated with immune complexes have shown an association with the presence and progression of atherosclerosis in both type 1 and type 2 diabetes. This is a promising approach which may help to better predict CVR not only in patients with diabetes, but also in other diseases with an accelerated development of atherosclerosis. However, additional studies conducted by different research groups on patients with different diseases or conditions associated with early atherosclerosis are needed to better define the implication of each type of modified LDL in the development of atherosclerosis.
Conflict of interestThe authors state that they have no conflict of interest.
The authors of this study have been funded by Instituto de Salud Carlos III (CIBERDEM, FIS PI05-2099, FIS CP06-0220 and FIS PI10-00265) and the Regional Government of Catalonia (2009-SGR-1205).
Please cite this article as: Sánchez-Quesada JL, Pérez A. Lipoproteínas modificadas como marcadores de riesgo cardiovascular en la diabetes mellitus. Endocrinol Nutr. 2013;60:518–528.