Iodine is considered to be an essential micronutrient in pregnant women. Iodine placental transport to the embryo–fetus is essential for hormone synthesis and is crucial for nervous system development. However, the relationship between iodine intake and placental weight and its potential implications for the newborn have not been studied.
Material and methodsIodine intake was analyzed in 77 pregnant women based on urinary iodine excretion (UIE) levels, measured using Pino's modified method (normal value, ≥150μg/L). Placental weight was measured (PW: normal, ≥500g). In the newborn, weight, height, and head perimeter (HP) were also measured. Placental index (PI: placental weight/newborn weight) was calculated, and was considered normal if ≥0.15.
ResultsUIE was normal in 50 pregnant women (mean±SD, 279±70.22μg/L) and decreased in 27 (94±31.49μg/L). Newborns of mothers with low UIE had a similar weight (3357±416.30g; n: 27) to those of mothers with normal UIE (3489±560.59g; n: 50). Forty-four percent of mothers with low UIE had PW <500g, and statistically lower HPs were found in newborns of mothers with low PW (PW3 500g: 36.05±0.55cm, n: 54; PW<500g: 33.93±15cm, n: 23, p<0.019). Similar results were found with PI, but they did not reach statistical significance (0.17±0.04; p=0.066). No differences were seen in all other parameters.
ConclusionThe study suggests the existence of a relationship between PW and HP. This finding may be related to iodine intake during pregnancy.
El yodo es un micronutriente esencial en la alimentación de la embarazada que transfiere al embrión-feto a través del transporte placentario. Existen antecedentes de su importancia para el desarrollo neurológico, pero no ha sido estudiada la relación entre ingesta de yodo y peso placentario ni su repercusión en el neonato (RN).
Materiales y métodosSe analizó ingesta de yodo en 77 embarazadas, mediante eliminación urinaria de yodo (EUI) con la técnica modificada por Pino (normal ≥150μg/l). Se midió el peso placentario (PP: normal ≥500g). En el recién nacido se evaluó peso, talla y perímetro cefálico (PC). Se obtuvo el índice placentario (IP: peso placentario/peso recién nacido) considerando normal ≥0,15.
ResultadosLa EUI fue normal en 50 embarazadas (media±DE, 279μg/l±70,22μg/l) y disminuida en 27 (94μg/l±31,49μg/l). Los RN de madres con EUI baja tenían un peso (3.357g±416,30g; n: 27) no diferente a las madres con yodurias normales (3.489g±560,59g; n: 50). Pero las madres con EUI bajo tenían un 44% de placentas con PP<500g y el análisis de los PC en los RN con bajo PP mostró que eran estadísticamente menores (PP3 500g: 36,05cm±0,55cm, n: 54; PP<500g: 33,93cm±15cm, n: 23, p<0,019). El estudio con los IP fue similar aunque no alcanzó la significación estadística 0,17±0,04 (p: 0,066). Los demás parámetros no mostraron diferencias significativas.
ConclusiónEl estudio evidencia una relación entre el PP y PC. Este hallazgo puede ser relacionado con la ingesta de yodo durante el embarazo.
The placenta is the organ allowing for the passage of inorganic iodine, a halogen included among the micronutrients which are important for thyroid hormone synthesis.1–3 Thyroid hormones play an essential role in the growth and development of fetal and extrauterine life through their regulation of the metabolic processes that promote body growth, heart function, lung maturation, and the development and differentiation of the central nervous system cells.4,5 During the first half of pregnancy, thyroid hormones used by the fetus are basically of a maternal origin, and the fetal thyroid gland gradually becomes able to synthesize its own hormones until it is able to guarantee all the thyroxine needed by the newborn at term.6,7 If pregnant women take less iodine than required, they may have low blood thyroxine levels, with the resultant negative impact on fetal brain development.8 Breast milk is the only iodine source for infants, and adequate iodine provision to breast-feeding women must therefore be ensured.9
There is no iodine pool in the body, and iodine must therefore be continuously replaced.10 Thyroid hormone requirement is increased during pregnancy,8,11 and the recommended daily intake of iodine by pregnant women and infants is therefore 200–300μg/day. It has been shown that in a pregnant woman, taking into consideration the dilution effect caused by increased urinary volume, the urinary iodine level is 166μg/L,12 which corresponds to the values considered normal.11
Iodine deficiency is recognized as the main cause of human disability that may be prevented by the mandatory iodination of table salt.13 It is known that in some regions, 38.7% of pregnant women have a low iodine intake. It is therefore essential to take action to ensure an adequate iodine supplementation of table salt.11
The WHO and the Panamerican Federation of Endocrinology Societies have requested that areas of endemic deficient iodine intake (DDI) should be determined, using for diagnosis urinary iodine excretion,11 thyroid ultrasound examination,9 and titration of iodine content in table salt.9,11 The WHO reported that 33% of countries have achieved analytical control of table salt. Spain is in group B, where the grade of DDI is still considered mild to moderate, because deficiency persists in some areas.14
There are no studies assessing UIE during pregnancy and its impact on placental weight (PW), and the impact of the latter on the anthropometric parameters of newborns. This study was therefore intended to assess whether a relationship exists between iodine deficiency and placental weight.
Materials and methodsAll pregnant women (77) attending the Departments of Endocrinology and Diabetes and Tocogynecology of Dr. Lucio Molas clinic in Santa Rosa, La Pampa, Argentine from March to August 2009 were enrolled into the study. Two urine samples (taken in the morning and evening) were requested in order to test urinary iodine excretion (UIE) using the Sandell Kholtoff method, as modified by Pino et al.15 The lower of the two values obtained was used for statistical analysis. Normal UIE was defined as 150μg/L, the value recommended by WHO/UNICEF/ICCIDD.11
In a term pregnancy (37–41 gestational weeks), the placenta has the shape of a circular or oval cake 18–20cm in diameter. Thickness is maximum (2.5cm) in the middle of the placenta, and lower at its margins. A median weight of 500g was assumed (P10: 280g and P90: 700g).16 Newborn and placental weights were controlled using a CoArMe scale. After delivery, the placenta was placed on an operating table, the membranes were removed, and the umbilical cord was sectioned at its insertion. The placenta was then washed with ice-cold physiological solution until all the fluid was drained, and the placental cake, excluding membranes and cord, was weighed. Maternal history during pregnancy (arterial hypertension, diabetes, and smoking) that could possibly determine high or low placental weight was recorded.
The weight and height of the pregnant woman were recorded, BMI (weigh/height2) was calculated, and patients were classified according to Atallah–Mardones as low weight, normal weight, overweight, or obese.16 The weight, height, and head perimeter (HP) of newborns were measured. Newborn weight was considered to be low if <2500g, normal if 2500–3999g, and high if ≥4000g. The ratio between newborn weight and placental weight, or placental index (PI), was calculated. A PI≥0.15 was considered normal.16
Variables were statistically analyzed using a Fisher's exact test and a two-tailed t test. Graphpad Prism version 4 for Windows was used for statistical analysis. Normality of variables was assessed using a Kolmogorov–Smirnov test. Data are given as mean±standard deviation (SD). A value of p<0.05 was considered statistically significant.
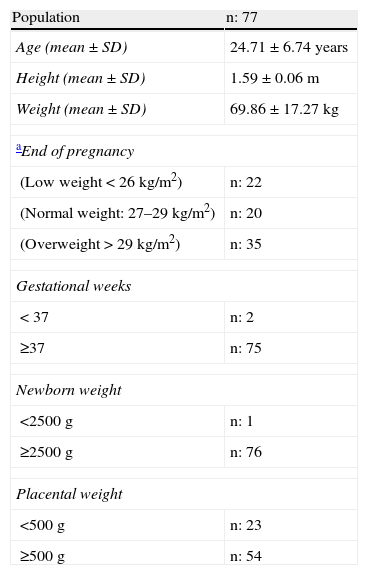
ResultsTable 1 shows the characteristics of the tested groups. Sixty-nine out of 77 pregnant women were older than 17 years, and ages ranged from 15 to 42 years (mean±SD, 24.71±6.74 years). Delivery was premature (before 37 weeks) in two of the 77 women, and at term in all other cases. Twenty-nine percent (22/77) of pregnant women had low weight and 26% (20/77) normal weight, while 45% (35/77) were overweight or obese. Twelve percent (9/77) of pregnant women had a history of gestational arterial hypertension. Of these, 1/9 had a PW<500g, 5/9 PWs ranging from 500 to 700g, to 3/9 PWs≥700g.
Description of study population.
| Population | n: 77 |
| Age (mean±SD) | 24.71±6.74 years |
| Height (mean±SD) | 1.59±0.06m |
| Weight (mean±SD) | 69.86±17.27kg |
| aEnd of pregnancy | |
| (Low weight<26kg/m2) | n: 22 |
| (Normal weight: 27–29kg/m2) | n: 20 |
| (Overweight>29kg/m2) | n: 35 |
| Gestational weeks | |
| <37 | n: 2 |
| ≥37 | n: 75 |
| Newborn weight | |
| <2500g | n: 1 |
| ≥2500g | n: 76 |
| Placental weight | |
| <500g | n: 23 |
| ≥500g | n: 54 |
Mardones: low weight <26.55, normal weight: 26.55–28.9, overweight and obesity >28.9.
There were no statistical differences in PW by weight, nutritional status, or gestational weeks of pregnant women. Among women with placentas weighing less than 500g, pre-term delivery occurred in 9% (2/23), as compared to 4% (2/54) of women with PWs≥500g (p: NS). There was a single case of low birth weight among the 23 newborns to women with PWs<500g.
Mean PW was 484.9±110.04g (mean±SD), with a range from 280 to 900g, while PI was 0.17±0.04. A single infant had a birth weight less than 2.500g, while 65/77 (84%) weighed 2500–4000g, and 11/77 (14%) ≥4000g. No weight or height differences were found between newborns from both groups.
Thirteen of the 77 pregnant women smoked at the start of pregnancy, but none of them could be considered as heavy smokers (more than 20 cigarettes daily). In the third trimester, only eight smoked occasionally. No association was found between smoking and study variables.
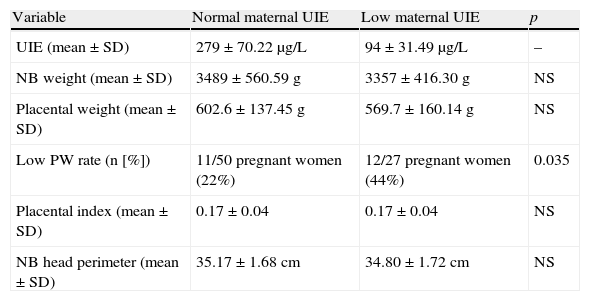
Table 2 analyzes UIE in relation to variables. Differences between urinary iodine levels (mean±SD; normal 279±70.22μg/L, n: 50; low: 94±31.49μg/L, n: 27) had no influence on PW, PI, or HP (Table 2). No significant differences in gestational age were found between the groups with normal and low UIE (data not shown).
Maternal and fetal variables related to urinary iodine excretion.
| Variable | Normal maternal UIE | Low maternal UIE | p |
| UIE (mean±SD) | 279±70.22μg/L | 94±31.49μg/L | – |
| NB weight (mean±SD) | 3489±560.59g | 3357±416.30g | NS |
| Placental weight (mean±SD) | 602.6±137.45g | 569.7±160.14g | NS |
| Low PW rate (n [%]) | 11/50 pregnant women (22%) | 12/27 pregnant women (44%) | 0.035 |
| Placental index (mean±SD) | 0.17±0.04 | 0.17±0.04 | NS |
| NB head perimeter (mean±SD) | 35.17±1.68cm | 34.80±1.72cm | NS |
UIE: urinary iodine excretion; NB: newborn; PW: placental weight.
Normal UIE n: 50.
Low UIE n: 27.
A statistically significant difference was however found when HP was analyzed in relation to PW (PW≥500g, HP: 36.05±0.55cm, n: 54; PW<500g, HP: 33.93±15.0cm, n: 23; p<0.019), and the same occurred with PI, although the significance was borderline in this case (PI≥0.15, HP: 35.78±4.05cm, n: 58; PI<0.15, HP: 34.32±1.80cm, n: 19; p=0.066).
When an attempt was made to analyze HP corrected for height, the nomogram was found to be insufficient to classify all the values found, and was therefore not used.
The results shown in Table 2 suggest that no relationship exists between UIE and HP, but careful analysis reveals that the frequency of PWs<500g is greater in pregnant women with decreased iodine excretion (normal UIE, 11/50 [22%]; 12/27 [44%], p<0.035), which suggests that in the study population, iodine intake could be a factor in low PW. This is supported by the 95% confidence and an odds ratio of 3 (1.06–8.5 times), meaning that mothers with PWs<500g have a three-fold greater rate of iodine deficiency, i.e. they have a three-fold higher statistical possibility of having a decreased UIE as compared to a pregnant woman with a PW≥500g.
DiscussionThe human placenta is the materno-fetal unit allowing for embryonic and fetal development through the oxygen supply and dialysis of fetal waste, nutrient transport, and endocrine and metabolic functions.1–4 Iodine is a micronutrient transported by the placenta which is used by the fetus to form thyroid hormones, the role of which is to promote somatic and neurological development.6–17
We attribute the lack of statistical correlation between PW and gestational weeks reported by other authors6–17 to the fact that 94.5% of pregnant women in this study had newborns at term, and gestational age is one of the main determinants of placental weight.6–17 The difference between term and pre-term newborns is probably due to the presence of a larger area of free-terminal chorionic villi and a higher number of blood vessels per villus.18 PWs were similar to those reported by Martina et al.19 in Peru in mothers with no risk factors. These authors argue19 that at a high altitude, PW is greater and NB weight lower as compared to sea level. Other authors refer to the placental vascular index as the ratio between vascular tree weight and PW.19 Both weights increase at high altitude, but it is not known which is predominant in this relationship. Placentas studied at high altitude have an increased placental vascularization as the result of hypoxia.19 Torry et al.20 attribute this to the fact that placental fetal capillaries are not static structures and may adapt themselves to a variety of changes and stress. Other authors think that a defective production of angiogenic growth factor is involved in intrauterine growth retardation, miscarriage, and pre-eclampsia.21
In our study, 25% of placentas analyzed had PIs<0.15, which was related to PWs<500g, thus validating the importance of this index when PW is lower as compared to newborn weight.19 Hindmarsh et al.22 suggest that newborn weight is related to placental weight in 41% and inversely related to multiparity and smoking in the mother during pregnancy.
We previously showed23 in a hospitalized population that 38.7% of pregnant women had low iodine intake, allowing for the early search and detection of thyroid dysfunction in these women. Thirty-five percent (n: 23) of the placentas assessed came from pregnant women with low iodine intake in some trimester of pregnancy, with a statistically significant relationship between low UIE and low PW, associated with a decreased HP as compared to placentas of greater weight. It could be hypothesized that smaller placentas would be associated with decreased nutrient supply and iodine transport leading to decreased fetal thyroid hormone synthesis.1–3 HPs found were within the 10th and 50th percentiles depending on the nomogram used and sex, but the statistical difference suggests a metabolic difficulty that should be corrected.
We agree with Hindmarsh et al.22 that placental weight determines infant growth as a continuous process. PW is therefore related to newborn weight and head perimeter, but in areas with chronic iodine deficiency, a lack of iodine should also be taken into account when considering placental development.
Conflict of interestThe authors state that they have no conflicts of interest.
We thank the Secretariat of Science and Technique of the UNLP School of Exact and Natural Sciences (research project no. 75). We also thank the heads of medical services and nursing staff of the Departments of Tocogynecology, Endocrinology and Diabetes, and Neonatology for their important, continued assistance. We acknowledge the biochemist Ricardo Fernandez Orsi and the laboratory technician Mariela Mayer for urinary iodine testing, and Dr. Rodolfo Rey for his statistical assistance.
Please cite this article as: Olivares JL, et al. Baja ingesta de yodo durante la gestación. Relación con el desarrollo placentario y el perímetro cefálico del recién nacido. Endocrinol Nutr. 2012;59:326–30.