Prolactinoma is the most frequent functioning pituitary adenoma. Most commonly occurs as microprolactinoma (less than 1cm in size), which may be cured with medical therapy, but few long-term studies are available about optimal duration of treatment with dopamine agonists to ensure cure after drug discontinuation and its withdrawal without recurrence are do not report consistent results.
ObjectiveTo establish criteria for cure of microprolactinoma with medical treatment and to analyze the potential predictors involved.
PatientsA retrospective study was conducted on 47 adult women with microprolactinoma followed up between 1975 and 2010; none of them had undergone prior surgery or radiotherapy, and all of them received treatment with a dopamine agonist for at least 4 years. They were divided into two groups for analysis: cured patients with at least 4 years with normal prolactin levels after drug discontinuation, and not cured patients.
ResultsCure was achieved in 57.4% of patients. Only age at diagnosis was a significant predictor: there were more young patients in the cured group and youngest patients needed less time to cure. Development of empty sella turcica or normal MRI was similar regarding time to cure.
ConclusionsMicroprolactinoma may be cured with dopamine agonists, and life-long treatment is not required, although more than 10 years may be required to achieve cure, 11.6±5.3 years in our experience.
El prolactinoma es el tumor hipofisario funcionante más frecuente. La mayoría son microprolactinomas (menos de 1cm), cuya curación es posible con tratamiento farmacológico, pero los estudios a largo plazo sobre la duración del tratamiento con agonistas dopaminérgicos para garantizar su curación tras suspender la medicación son escasos y no ofrecen consenso al respecto ni sobre criterios de retirada del tratamiento.
ObjetivoEstablecer criterios de curación del microprolactinoma con tratamiento médico y analizar los posibles factores implicados.
PacientesSe revisaron 47 mujeres con microprolactinomas tratadas exclusivamente con agonistas dopaminérgicos durante al menos 4 años, entre 1975 y 2010. Se compararon las curadas, con más de 4 años con prolactina normal sin medicación, y las no curadas.
ResultadosSe alcanzó la curación en el 57,4% de los casos, tras un tiempo medio de tratamiento de 11,6 años. Entre las variables analizadas, solo la edad al diagnóstico resultó significativa como factor pronóstico: las pacientes más jóvenes curaron en mayor proporción y en menos tiempo. Entre los curados, 6 evolucionaron a silla turca vacía parcial y en 21 desapareció el adenoma (RM normal), sin observarse diferencias en ambos subgrupos.
ConclusionesEl microprolactinoma puede curarse con agonistas dopaminérgicos y el tratamiento no ha de mantenerse por vida, aunque se pueden requerir más de 10años para alcanzar la curación.
Prolactinomas are prolactin (PRL)-producing tumors that comprise around 40% of all pituitary hormone-secreting neoplasms in adults. Up to 90% of them are microprolactinomas. They can be dealt with medically, which is the treatment of first choice, surgically, or with radiotherapy. Prolactinomas are classified according to their diameter1 in microprolactinomas (below 1cm, intrasellar) and macroprolactinomas (equal to or above 1cm).2 The latter can be further classified according to their clinical behavior as non-invasive if they are confined to the sella turcica, or as invasive if they affect surrounding structures. Microprolactinomas are more frequent among women, while macroprolactinomas are usually found in men.3
In general, microprolactinomas grow slowly and rarely turn into macroprolactinomas, while macroprolactinomas, on the other hand, usually increase in size progressively,4 which suggests an inherently different type of behavior between the two types of tumors.
The goals of treatment are to restore gonadal function and control symptoms, normalize PRL levels, reduce tumor size, inhibit tumor growth, and to prevent the involvement of surrounding structures.5
Dopamine agonist (DA) therapy is the first-line treatment approach for the management of prolactinomas, regardless of tumor size. It is effective in the majority of cases, except for some patients in whom surgery and/or radiotherapy is necessary because of insufficient DA-response. These are two relatively complicated approaches and are not always curative. Furthermore, they are both associated with high recurrence rates6 and may entail undesirable effects such as hypopituitarism, so they are usually reserved for cases in which conventional medical treatment is insufficient.
Bromocriptine was the first DA to be used for the management of hyperprolactinemia and, because it has not been associated with adverse effects on fetal development, it is the only one approved for the treatment of prolactinomas in pregnancy.1,4 The other two most frequently used DAs are cabergoline and quinagolide. All three are effective for the normalization of PRL levels and the restoration of gonadal function, and they are even curative after several years of treatment. However, their long term efficacy has not been fully evaluated.
This study aims to describe the main clinical characteristics and long-term results regarding the symptoms, tumor size and possible cure of a series of patients with microprolactinomas treated exclusively with DA therapy. We will consider the criteria for cure and we will analyze the optimal treatment duration to achieve it without recurrence after the withdrawal of medication. Furthermore, we aim to elaborate a clinically practical algorithm for the management and follow-up of microprolactinomas using DA therapy, and to investigate which variables may determine an effective therapeutic response to these drugs.
Materials and methodsWe conducted a retrospective study of a series of 82 patients with prolactinomas who were followed-up in our center from 1975 to 2010. Macroprolactinomas, cases who had been treated with surgery or radiotherapy, and those individuals with less than 2 years of follow-up were excluded from the analysis, leaving a total of 57 patients with microprolactinomas, all of whom were women. Given the fact that none of them could be considered as cured before a period of 4 years, we further excluded 10 more patients who had been medically treated for less than 4 years and were not cured, so the final sample comprised a total of 47 women.
Criteria for treatment withdrawal- -
Resolution of symptoms (amenorrhea, galactorrhea, headache, etc.).
- -
Disappearance of pituitary tumor: normal magnetic resonance imaging (MRI) or primary empty sella (PES).
- -
PRL levels <20ng/mL, with an adequate DA dose, during a period of at least 2 years.
- -
Progressive tapering of DA dose until total withdrawal, after one year in which PRL levels were maintained <10ng/mL with the minimum DA dose.
- -
Cure was defined as the persistence of the above-mentioned characteristics (with PRL levels <20ng/mL) for a period of at least 2 years after DA withdrawal.
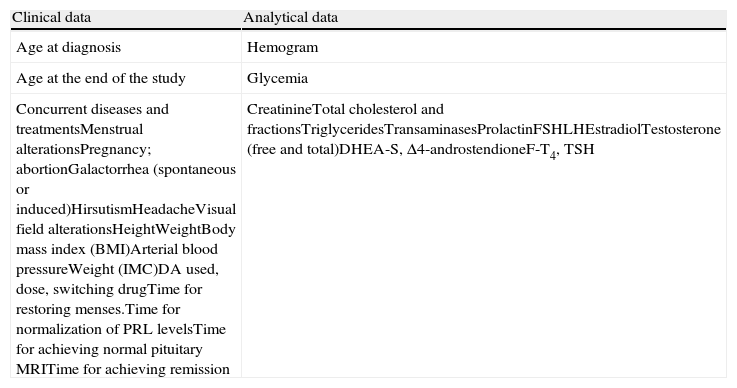
Table 1 summarizes the clinical and analytical data collected in our study. Pituitary morphological evaluation was performed using pituitary MRI before, during and after the withdrawal of treatment with DA therapy. Pituitary MRI was performed using a high field-strength magnet (1.5T unit), with 7–11, 2–3mm slices, and a spatial resolution of 0.70–0.97mm, before and after gadolinium administration. Images were classified as normal pituitary, intrasellar tumor remnant, or PES.
Clinical and analytical variables recorded.
| Clinical data | Analytical data |
| Age at diagnosis | Hemogram |
| Age at the end of the study | Glycemia |
| Concurrent diseases and treatmentsMenstrual alterationsPregnancy; abortionGalactorrhea (spontaneous or induced)HirsutismHeadacheVisual field alterationsHeightWeightBody mass index (BMI)Arterial blood pressureWeight (IMC)DA used, dose, switching drugTime for restoring menses.Time for normalization of PRL levelsTime for achieving normal pituitary MRITime for achieving remission | CreatinineTotal cholesterol and fractionsTriglyceridesTransaminasesProlactinFSHLHEstradiolTestosterone (free and total)DHEA-S, Δ4-androstendioneF-T4, TSH |
After the withdrawal of treatment, MRI was repeated every 6 months during the first year and yearly thereafter.
Statistical analysisCategorical variables are presented as frequencies. Quantitative variables are expressed as mean, standard deviation (SD), minimum and maximum. For variables which did not follow a normal distribution, their median and interquartile range are described.
The association between categorical variables was assessed using Pearson's chi-square test (χ2) (Fisher's exact test as required). Quantitative variables were evaluated for each independent categorical variable using Student's t-test (one-variable comparisons with 2 categories).
The p values were two-sided and statistical significance was considered when p<0.05. All statistical analyses were performed using SPSS version 15.0 (IBM SPSS Statistics Inc., Chicago, IL, USA).
ResultsAge at diagnosis was 29.9±8.3 (16–45) years and baseline serum PRL levels were 129.5±87.8 (33–522)ng/mL. The follow-up period was 16.2±8.0 (4–35) years. Medical treatment was prescribed for a period of 12.8±6.7 (2–27) years.
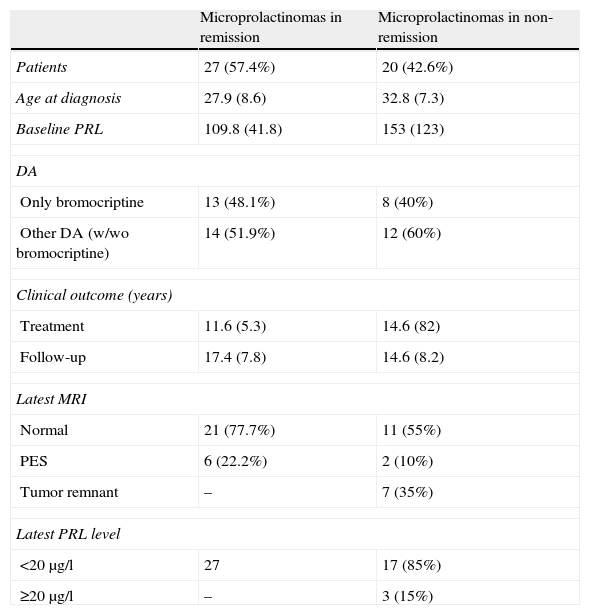
For the remaining analyses, results are expressed separately for cured and not cured women, as shown in Table 2.
Results.
| Microprolactinomas in remission | Microprolactinomas in non-remission | |
| Patients | 27 (57.4%) | 20 (42.6%) |
| Age at diagnosis | 27.9 (8.6) | 32.8 (7.3) |
| Baseline PRL | 109.8 (41.8) | 153 (123) |
| DA | ||
| Only bromocriptine | 13 (48.1%) | 8 (40%) |
| Other DA (w/wo bromocriptine) | 14 (51.9%) | 12 (60%) |
| Clinical outcome (years) | ||
| Treatment | 11.6 (5.3) | 14.6 (82) |
| Follow-up | 17.4 (7.8) | 14.6 (8.2) |
| Latest MRI | ||
| Normal | 21 (77.7%) | 11 (55%) |
| PES | 6 (22.2%) | 2 (10%) |
| Tumor remnant | – | 7 (35%) |
| Latest PRL level | ||
| <20μg/l | 27 | 17 (85%) |
| ≥20μg/l | – | 3 (15%) |
DA: dopamine agonist; B: bromocriptin; PRL: prolactin; PES: primary empty sella.
In the group of women who were not cured, 12 patients fulfilled remission criteria, but their treating physicians chose to continue DA treatment, so they were included under this category.
Women in the remission category were younger than women who were not cured (27.9±8.6 vs 32.8±7.3 years, p=0.073), but we did not find any significant differences among the other characteristics evaluated.
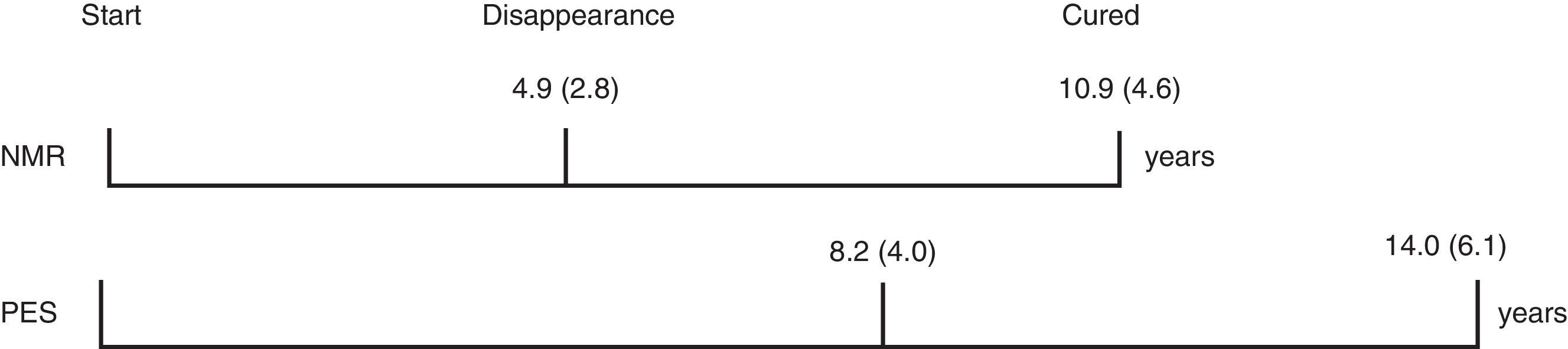
In the group of cured women, a trend for a longer duration of treatment prior to adenoma reduction or PES was observed (8.2±4.0 vs 4.9±2.8 years), but the subsequent time for resolution was similar in both subgroups (5.9 and 5.8 years, respectively, Fig. 1). Cured patients with normal pituitary MRI did not progress to PES.
DiscussionThe optimal duration of DA treatment for microprolactinomas is currently a matter of debate and several experts consider that lifetime DA prescription is necessary. In fact, a survey performed among endocrinologists in the United Kingdom7 observed that 20% of them never attempted to withdraw treatment.
Numerous reports have published data regarding the efficacy of DA treatment for controlling symptoms, but they have not fully assessed remission rates and subsequent follow-up. Webster et al.8 reported prolonged treatment with cabergoline in 162 cases, but their observations were limited to a 7-month follow-up period. Verhelst et al.9 studied 455 patients with microprolactinomas who were also treated with cabergoline during a period of 28 months, but it was a study whose main purpose was to evaluate tolerance and efficacy, and cabergoline was switched to bromocriptine in cases in which intolerance or resistance to treatment was observed. Our study only evaluated microprolactinomas in 47 women, but the period of observation lasted for 16.2±8.0 years.
One of the largest series of patients is the one described by Webster et al.,10 with a total of 459 cases, but it only compared the efficacy of cabergoline and bromocriptine for a period of 6 months. Passos et al.11 reported 131 patients treated with bromocriptine exclusively for 47 months. They observed that 64% of microprolactinomas remained in remission 44 months after treatment withdrawal. Colao et al.12 evaluated 200 patients with hyperprolactinemia (105 microprolactinomas) who received cabergoline treatment for a mean period of 4 years, and they observed a remission rated of 69%.
Several criteria addressing the optimal time for treatment withdrawal have recently been proposed. For instance, Colao et al.12 suggest keeping treatment with cabergoline for 36–48 months. They discontinue treatment once PRL levels are normal, and one year after obtaining a negative pituitary MRI, or a reduction in tumor size of at least 50%. The Pituitary Society's experts committee advocates a minimum 3-year duration of treatment, with normal PRL levels under DA treatment, and a significant tumor reduction, before withdrawing medication.
Other publications4,12–14 point out that a normal pituitary MRI 1 year after treatment removal is not enough to avoid the recurrence of microprolactinomas, and they suggest that DA therapy should be maintained for at least 2 years after no tumor remnant is visible or a significant reduction of at least 50% has been verified.
The clinical manifestations usually disappear a couple of weeks after initiating DA therapy, but this clinical normalization does not necessarily imply a definitive cure, since a recurrence of symptoms may occur 2–3 weeks after treatment has been stopped. It seems reasonable therefore to closely monitor pituitary imaging at least annually, and to refrain from attempting to withdraw treatment until the tumor remnant has disappeared.
Colao et al.12 maintained DA treatment for a mean period of 4 years; in their series, a recurrence of hyperprolactinemia was observed in 30% of patients, even in some cases in whom there was no evidence of pituitary tumor, although a relapse was more frequent in patients with a tumor remnant at the time of DA withdrawal.
In our series, a mean time of 6 years was necessary to achieve a normalization of pituitary MRI or the development of PES, and fulfill remission criteria. This would favor the recommendation of continuing DA treatment for at least 2–3 years after no tumor remnant is visible in pituitary MRI. Therefore, we consider that PES does not necessarily entail the remission of microprolactinoma,15,16 and that this is confirmed by the fact that PES was observed in 2 out of the 20 cases that were not considered as cured.
Colao et al.12 suggested that if tumor reduction of >50% was observed, DA treatment could be stopped. However, later, her group4 and other authors1,13,14 remarked that waiting for 1 year after the normalization of MRI was not enough to achieve persistent remission, and recommended that treatment should be continued for a further 2 or 3 years. Arafah and Nasrallah17 encourage waiting for at least 5 or 6 years before attempting to withdraw treatment.
Biswas et al.7 reported a remission rate of 36% among 89 patients treated with cabergoline or bromocriptine during a mean period of 3.1 years, and they also suggested that the remission rates observed by Colao et al.12 were overestimated. In the study by Kharlip et al.13 which described the evolution of 31 microprolactinomas, recurrence rates reached 52% 15 months after treatment with cabergline was withdrawn after having been prescribed for 3.6 years. Half of the patients in remission were followed up for less than 15 months and 64% of them had persistent tumor remnant, which depicts a higher failure rate. In a recent meta-analysis, Dekkers et al.18 reviewed 19 publications which included a total of 743 patients with hyperprolactinemia. They found that remission was achieved in 21% of microprolactinomas and suggested a minimum of 3–5 years of continued DA treatment.
In our series, age and PRL levels at the time of diagnosis of hyperprolactinemia were similar to the ones reported by others.7,11,13 Younger women attained MRI normalization sooner, were less prone to developing PES and fulfilled remission criteria 3 years earlier than older women.
Most of our patients received treatment with bromocriptine, because this is the oldest drug used for the medical management of prolactinomas. When routine regimes were switched to cabaergoline, remission was not achieved earlier and the duration of treatment was longer than for those on bromocriptine. This could be interpreted as being due to the greater efficacy of bromocriptine, but it must be noted that these patients were also younger. Furthermore, it could also be inferred that these women exhibited a greater resistance to bromocriptine, which determined the need for a longer duration of treatment: a “non-curative” one with bromocriptine, and a “curative” treatment with cabergoline.
This is in contrast with previous publications which address the superiority of cabergoline in comparison to bromocriptine regarding the control of hyperprolactinemia and tumor shrinkage.4,12,14 However, we believe that due to the retrospective nature of our study, we are in no position to arrive at definitive conclusions concerning direct comparisons between the two drugs.
Menopause was observed in 6 patients with normal MRI and in 3 with persistent tumor remnant; this confirms that menopause is not necessarily a prognostic factor determining remission, as has also been remarked on by others.11,12
We did not observe any significant differences in baseline PRL levels between patients who achieved remission and those who did not, in agreement with some previous reports,4,11–13,17 but in contrast to others.7
Some studies4 have included under the category of microprolactinomas pituitary images in which no tumor was observed. However, we believe this to be a confounding issue and, therefore, we have not considered this possibility as one of our inclusion criteria. In our experience, cases with hyperprolactinemia and negative sellar imaging are frequently due to the concomitant use of drugs, and not to microprolactinomas. In this way, “spontaneous remission” could be actually attributed to withdrawal of the drug itself.
Complete normalization of pituitary imagining does not seem to be a necessary criterion for defining remission. It is however advisable that at least a 50% volume reduction and normalization of PRL levels should be achieved with minimal doses of DA before any attempt is made to withdraw medical treatment.
ConclusionsIn this study, we elaborate clearer and more straightforward indications for withdrawing medical treatment for microprolactinomas, and we specify the criteria which define remission. Pituitary imaging plays an important role, although its limitations regarding sensitivity should be acknowledged, and a normalization or development of PES does not unequivocally ensure a complete and persistent cure. In selected patients without symptoms and low levels of PRL with minimal doses of DA, persistent tumor remnant imaging could really be due to fibrous tissue with no clinical relevancy.
Life-long treatment is not always necessary, although in some cases prolonged regimes may be required. In our experience, remission was achieved in 57.4% of patients after maintaining DA for a mean period of 11.6±5.3 years. None of the women included in our study experienced confirmed remission before 4 years.
Regarding the clinical characteristics associated with remission, younger age was the only factor which was observed to influence remission rates.
Two years without clinical symptoms and normal PRL levels after treatment withdrawal are reasonable criteria for defining remission. However, long-term monitoring will still be required as recurrence has been described as occurring even after 3 years.
Conflict of interestThe authors declare no conflict of interest.
Please cite this article as: Martín de Santa-Olalla y Llanes M, Andía Melero VM, Jara Albarrán A. Evolución del microprolactinoma a largo plazo con tratamiento médico. Endocrinol Nutr. 2013;60:489–494.