Liver abscess (LA) is a pus collection located in the liver resulting from any infectious process that causes the destruction of liver parenchyma and stroma. LAs are, in order of frequency, of pyogenic (or bacterial), fungal, and amebic origin.
Pyogenic LA (PLA) may occur by contiguity, as a complication of a biliary tract or abdominal infection, by hematogenous dissemination, or may be cryptogenic (in up to 25–60% of cases). PLAs generally contain multiple microorganisms, which usually come from the gastrointestinal flora. Various recent reports have suggested an increased incidence of PLAs in the elderly, cancer patients, and immunosuppressed subjects.1 In a recent study conducted in the United Kingdom, Mohsen et al. have found an annual incidence of 18 cases per 100,000 admissions.2 In Spain, the annual incidence is 14–35 cases per 100,000 admissions.3
Escherichia coli has traditionally been considered as the main agent responsible for PLA. LAs caused by Klebsiella pneumoniae (K. pneumoniae) were initially reported in Eastern countries,4 but cases of such infection have been reported in recent years in Western countries, including Spain. In addition, according to some studies, its incidence is gradually increasing.5 The vast majority of LAs induced by Klebsiella spp. are caused by K. pneumoniae.6,7
Diabetes mellitus (DM) has been shown to be the most significant risk factor for developing a LA caused by K. pneumoniae.6 We report a case of hyperglycemic decompensation in a young patient which was triggered by a LA caused by K. pneumoniae with hematogenous dissemination and pulmonary embolism.
This was a 46-year-old male taken to the emergency room after being found stuporous, febrile, and with hyperglycemia at his home. For the previous two days, the patient had had a dry cough, low-grade fever, self-limited vomiting, and urinary frequency. His personal history included post-pancreatectomy diabetes mellitus diagnosed 10 years before an episode of alcoholic pancreatitis. No biliary stones or other biliary or pancreatic condition was found. The patient had chronic poor glucose control with glycosylated hemoglobin levels ranging from 9% to 12%, and had been admitted to hospital six months before for diabetic ketoacidosis. There were no known chronic complications of diabetes, and the patient did not regularly attend a medical clinic. Kidney function was normal, with creatinine levels ranging from 0.5 to 0.9mg/dL. The patient also had high blood pressure, dyslipidemia, hypothyroidism, and schizophrenia. He was a smoker and a sporadic consumer of cannabis, and drank 50–90g of alcohol daily. His usual treatment included premixed insulin lispro/NPL (25/%75%), 20 and 16 UI before breakfast and dinner, respectively, atorvastatin 10mg/day, losartan 100mg/day, levothyroxine 50 mcg/day, clorazepate dipotassium 5mg/day, omeprazole 20mg/day, and pancreatin 300mg 3 times daily.
A physical examination showed a fair general status, pallor, dry mucosal membranes, sweating, blood pressure values of 107/54mmHg, a temperature of 39°C, a heart rate of 101bpm, and 96% oxygen saturation. Cardiac auscultation was normal, and pulmonary auscultation revealed scattered rhonchi. The physical examination was otherwise unremarkable.
Blood laboratory test results included: glucose 501mg/dL, urea 122mg/dL (normal range [NR], 17–43), creatinine 1.83mg/dL (NR, 0.67–1.17), sodium 119mEq/L (NR, 136–146), potassium 3.6mEq/L (NR, 3.5–5.1), C-reactive protein 14 (NR, 0.0–0.5), WBC 12,200 (94.1% neutrophils), and hemoglobin 10.6g/L. Urine analysis revealed maximal ketonuria and glycosuria. Venous gas values included pH of 7.47 and bicarbonate 19.7. Serologic testing for hepatitis B and human immunodeficiency virus was negative. Chest W-rays showed a predominantly peripheral nodular pattern. Blood cultures showed a growth of K. pneumoniae at 24 and 48h.
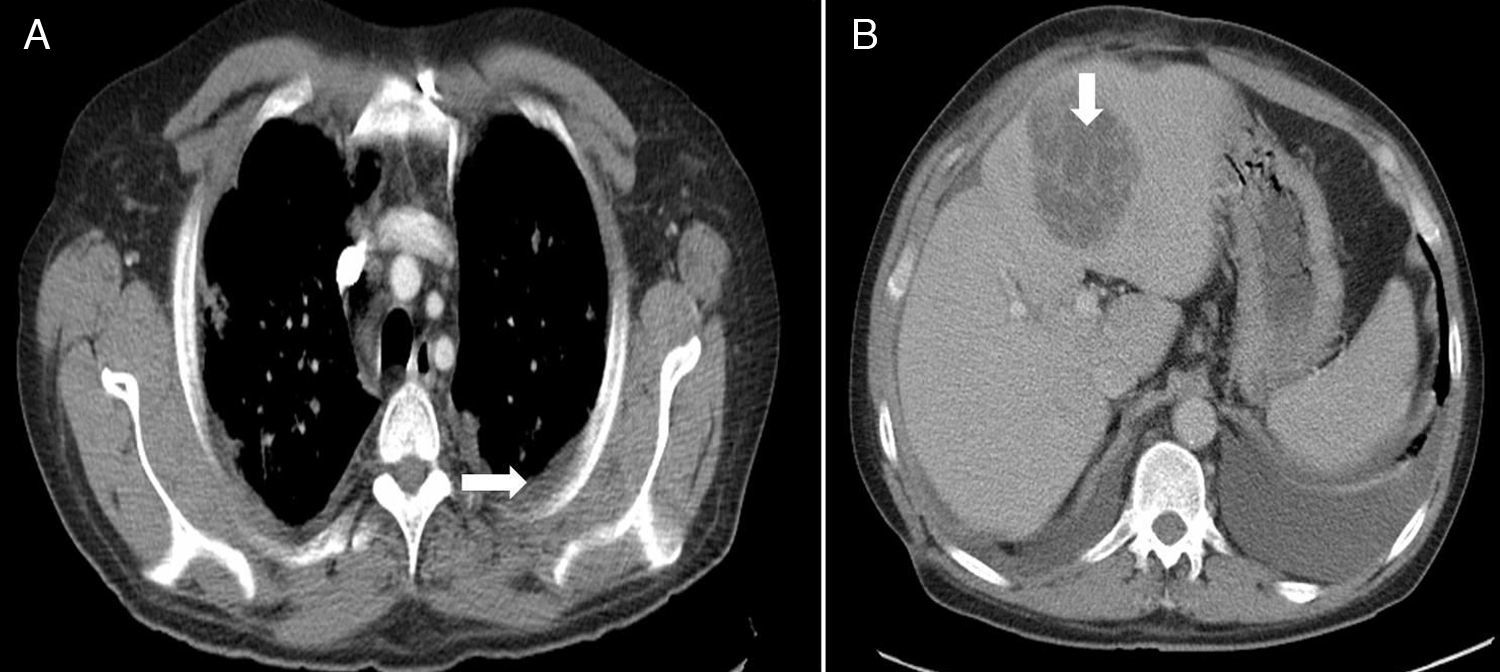
Treatment was started with fluid therapy and insulin infusion, as well as a broad-spectrum antibiotic (piperacillin-tazobactam). An echocardiogram was normal. Computed tomography (CT) of the chest and abdomen showed left pleural effusion, diffuse interstitial bilateral infiltrates, and predominantly peripheral bilateral nodules in the chest, and a hypodense hepatic lesion 6cm×7cm in diameter in the abdomen (Fig. 1).
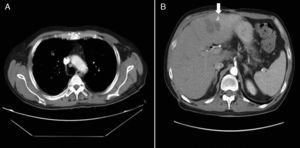
Kidney failure was managed with hydration and blood glucose correction, and was considered to be of prerenal origin. However, the patient had a torpid course with partial respiratory insufficiency. Fibrobronchoscopy, thoracocentesis, and pleural fluid analysis showed no changes. CT-guided puncture and drainage of the hepatic abscess were performed. A culture of drained hepatic material showed a growth of K. pneumoniae. Adult respiratory distress syndrome and septic pulmonary embolisms secondary to hepatic abscess caused by K. pneumoniae were diagnosed. Antibiotic therapy was switched to amikacin, linezolid, and metronidazole. The patient improved 48h after percutaneous drainage, with normalization of respiratory function. He was subsequently discharged from hospital on oral antibiotic therapy consisting of ciprofloxacin 500mg every 12h and metronidazole 500mg every 8h for 4 weeks. A CT of the chest and abdomen performed one month after discharge showed radiographic improvement as compared to the previous one, including the disappearance of bilateral laminar pleural effusion. A hypodense image, smaller than in the prior CT, persisted in the left liver lobe (segment 3) (Fig. 2).
LA usually poses a diagnostic challenge, as laboratory data are non-specific, and classical clinical signs include fever, chills, pain in the right hypochondriac region with or without hepatomegaly and jaundice. The reported patient had severe hyperglycemic decompensation, with ketosis, mild compensated metabolic acidosis, non-specific radiographic and clinical pulmonary changes, and mild leukocytosis.
The febrile peak at admission suggested an infectious site, but the other clinical signs did not suggest the final diagnosis. LA is a potentially serious condition, despite the fact that prognosis has improved in recent years. A review of cases of pyogenic liver abscess reported a 2.5% mortality rate, in contrast with the 11–31% mortality rates reported by other studies.7 LAs induced by K. pneumoniae have a better prognosis than polymicrobial abscesses, with less recurrence (4.3% versus 41%) and mortality.6
Once cultures have been shown to be positive for K. pneumoniae, adequate antibiotic therapy should be started based on susceptibility tests, and the source of the primary infectious process should be sought.
According to the most recent clinical reviews reported, DM is the main risk factor for pyogenic abscesses caused by K. pneumoniae serotype K1.6 An impaired phagocytosis in Kupffer cells in diabetic subjects has been postulated as a pathophysiological hypothesis. Treatment of LA caused by K. pneumoniae includes specific antimicrobial therapy for 4–6 weeks combined with percutaneous drainage, which has been shown to be the most effective treatment.7
Paradoxically, DM has not been shown to be a mortality predictor, while gas formation in the abscess and creatinine levels higher than 1.3mg/dL are mortality predictors.8 LA caused by K. pneumonia in nondiabetic subjects usually occurs after surgery for any abdominal condition, especially gastrointestinal. In diabetic patients, however, LA characteristically occurs without prior biliary and gastrointestinal disease.6 A patient history of pancreatectomy 10 years before is very unlikely to have any relationship to LA. A single study has shown that, in addition to DM, chronic alcohol consumption may be a risk factor for LA caused by K. pneumoniae.9 Diabetic patients do not usually have typical symptoms suggesting this condition such as abdominal pain, fever, leukocytosis, or increased transaminase levels.8 Our patient had experienced prior low grade fever and an isolated febrile peak upon admission. Leukocytosis was poorly significant and is also a common finding in the event of ketoacidosis.
Metastatic foci have been found in 12% of diabetic subjects with LA caused by K. pneumoniae.5 The most common include endophthalmitis, lung abscesses, meningitis/brain abscesses, bacteriuria/prostatic abscesses, and psoas abscesses. Some studies have reported lung involvement in 100% of patients.10 CT of the chest, abdomen and pelvis, an echocardiogram, urinary analysis, and fibrobronchoscopy were performed on the patient to rule out other foci of metastatic abscesses. A brain CT was not requested, because he had no neurological symptoms and an outpatient bone scan showed no involvement.
In conclusion, although LA caused by K. pneumoniae is an uncommon condition, it should be taken into account in clinical practice because DM is the main predisposing factor, particularly in poorly controlled or especially immunocompromised subjects. Although mortality is not negligible, adequate early diagnosis and treatment allows for a favorable course.
Please cite this article as: Rodríguez-Lagos FA, et al. Absceso hepático por Klebsiella pneumoniae en un paciente diabético. Endocrinol Nutr. 2013;60:106–9.