Some recent studies have related autoimmune thyroid dysfunction and gestational diabetes (GD). The common factor for both conditions could be the existence of pro-inflammatory homeostasis. The study objective was therefore to assess whether the presence of antithyroid antibodies is related to the occurrence of GD.
Material and methodsFifty-six pregnant women with serum TSH levels≥2.5mU/mL during the first trimester were retrospectively studied. Antithyroid antibodies were measured, and an O'Sullivan test was performed. GD was diagnosed based on the criteria of the Spanish Group on Diabetes and Pregnancy.
ResultsPositive antithyroid antibodies were found in 21 (37.50%) women. GD was diagnosed in 15 patients, 6 of whom (10.71%) had positive antibodies, while 9 (16.07%) had negative antibodies. Data were analyzed using exact logistic regression by LogXact-8 Cytel; no statistically significant differences were found between GD patients with positive and negative autoimmunity (OR=1.15 [95%CI=0.28–4.51]; P=1.00).
ConclusionsThe presence of thyroid autoimmunity in women with TSH above the recommended values at the beginning of pregnancy is not associated to development of GD. However, GD prevalence was higher in these patients as compared to the Spanish general population, suggesting the need for closer monitoring in pregnant women with TSH levels≥2.5mU/mL.
Recientemente varios trabajos han relacionado la disfunción tiroidea autoinmune con la diabetes gestacional (DG). El hipotético nexo de unión sería el desarrollo de la homeostasis proinflamatoria. Por ello nos propusimos estudiar si la presencia de anticuerpos antitiroideos se relaciona con la aparición de DG.
Material y métodosSe estudiaron retrospectivamente 56 gestantes con valores de TSH≥2,5μU/mL en el primer trimestre. Se midieron anticuerpos antitiroideos y se realizó la prueba de O'Sullivan. Para el diagnóstico de DG se llevó a cabo una sobrecarga oral de glucosa (100g) y se siguieron los criterios recomendados por el Grupo Español de Diabetes y Embarazo.
ResultadosSe constató anticuerpos antitiroideos elevados en 21 (37,50%) mujeres. Se diagnosticó DG en 15 (26,79%) pacientes, de las que 6 (10,71%) tenían anticuerpos positivos y 9 (16,07%) tenían anticuerpos negativos. Los datos fueron analizados mediante regresión logística exacta por LogXact-8 Cytel, no encontrándose diferencias significativas entre las pacientes diagnosticadas de DG con anticuerpos antitiroideos positivos y con autoinmunidad negativa (OR=1,15 [IC 95%=0,28–4,51]; P=1,00).
ConclusionesLa presencia de autoinmunidad tiroidea en mujeres con TSH por encima de los valores recomendados al inicio de la gestación no se asocia con el desarrollo de DG. No obstante, la prevalencia de DG en estas pacientes es superior a la documentada en la población general española, lo que sugiere la necesidad de un seguimiento más estrecho en gestantes con TSH≥2,5μU/mL.
Gestational diabetes (GD) is defined as any grade of carbohydrate intolerance first diagnosed during pregnancy.1 Documented GD prevalence ranges from 1% and 14%.1,2 GD prevalence in our environment is approximately 10%.3–6
It is estimated that GD is caused by β-cell dysfunction in most patients. The underlying mechanism is increased insulin resistance with no decrease in insulin secretion.7 Some studies have documented a high prevalence of elevated thyroid antibodies in situations where background insulin resistance predominates, such as pregnant women with GD,8 patients con type 2 diabetes mellitus,9 or in polycystic ovary syndrome.10 It is therefore speculated that thyroid autoimmunity could be a risk factor for diabetes. The link between autoimmune disorder and insulin resistance could be the inflammatory events associated to both conditions. Thus, for example, serum cytokine levels are increased both in patients with thyroid autoimmunity11 and insulin resistance.12 It has also been reported that patients with subclinical hypothyroidism of an autoimmune origin show increased levels of C-reactive protein which do not change with thyroid function status.13 This characteristic may be parallel to increases in C-reactive protein reported in prediabetic subjects with autoimmunity against pancreatic islet cells.14 In addition, presence of inflammatory markers has been related to diabetes mellitus in pregnant women,15,16 and thyroid dysfunction has also been related to insulin resistance.17–19
Because of this potential connection between autoimmune thyroid dysfunction and insulin resistance, we decided to analyze the potential relationship between autoimmunity in pregnant women with hypothyroidism and its relationship to GD.
Patients and methodsPopulationA retrospective evaluation was made of 56 women (age: 32.89±4.16 years; mean±SD) referred to our department from 2008 to 2012 for TSH levels ≥2.5μU/mL at the start of pregnancy, according to the recommendations of the American Thyroid Association (ATA).20 Age (years), pregestational body mass index (BMI) (kg/m2), and family history of diabetes mellitus was recorded in all patients. Patients with personal history of impaired carbohydrate metabolism and prior diagnosis of thyroid dysfunction were ruled out.
All patients were measured thyroid-stimulating hormone (TSH) levels and thyroid peroxidase (anti-TPO) and thyroglobulin (anti-TG) antibodies during the first trimester of pregnancy. After diagnosis of hypothyroidism, all pregnant women were treated with l-thyroxine, and their thyroid function was monitored by monthly tests of thyroid function until delivery. Antibodies were usually measured 1–4 weeks after diagnosis of hypothyroidism, after starting treatment with l-thyroxine supplements.
On week 24–28 of pregnancy, all patients were screened for gestational diabetes using the O'Sullivan test according to the standard protocol. A positive test was defined as a blood glucose level greater than 140mg/dL 1h after oral administration of 50g of glucose. Study was completed in these patients by administering an oral glucose overload of 100g after fasting for 12h. Diagnosis was made using the criteria of the Third workshop, as recommended by the Spanish Group on Diabetes and Pregnancy.21
Patients with TSH levels≥2.5μU/mL in whom diagnosis of GD was negative were used as controls.
MethodsTSH was measured using electrochemiluminescence immunoassay (Modular Analytics E170; Roche); RV: 0.38–4.7μU/mL. During the first trimester of pregnancy, a value ≥2.5μU/mL was considered as pathological. TPO antibodies were measured by ELISA (QUANTA Lite®); RV<29IU/mL, and TG antibodies by chemiluminescence immunoassay (Access® 2 Immunoassay System; Beckman Coulter); RV<4IU/mL Glucose levels were measured using a glucose oxidase reaction (UniCel DxC 800; Beckman Coulter).
Statistical studyThis was a convenience sample consisting of patients who met the established criteria, with no prior sample size estimation.
A Student's t test was performed to compare the means of quantitative variables between the GD and control groups, verifying normal distribution by the Kolmogorov–Smirnov and Shapiro–Wilk tests. Frequencies of qualitative variables were compared using a Pearson's Chi-square test, or a Fisher's exact test when one of the absolute frequencies was less than 5. The strength of association of risk factors (thyroid antibodies) to GD occurrence was calculated by estimating the odds ratios (OR) and 95% confidence intervals (CIs) by a logistic regression analysis using LogXact-8 Cytel software. Confidence levels higher than 95% (P<0.05) were considered significant in all cases. Data are shown as absolute (n) or relative (%) frequencies and as means with their standard deviation (SD).
ResultsAt diagnosis of hypothyroidism (TSH ≥2.5μU/mL) during the first trimester of pregnancy, mean TSH level was 4.60±1.77μU/mL. Thyroid autoimmunity was found in 21 patients (37.50%). Nineteen patients (33.92%) had TPO antibodies, and 7 patients (12.50%) TG antibodies. Five women (8.93%) had both types of antibodies.
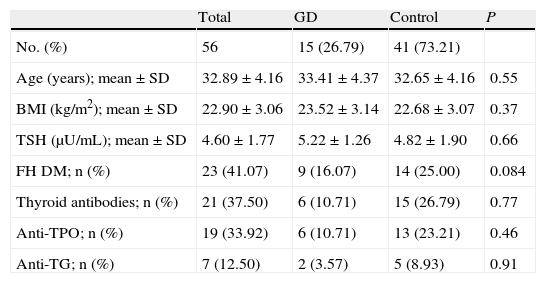
Twenty-one women (37.50%) of the sample had a positive O'Sullivan test, and 15 of these (26.79%) were finally diagnosed with GD. No statistically significant differences were found in age, body mass index, antibody titer. or TSH levels at the start of pregnancy, nor in family history of diabetes mellitus, between pregnant women diagnosed with GD and the control group (Table 1).
Demographic characteristics of patients with and without gestational diabetes at the start of pregnancy.
| Total | GD | Control | P | |
| No. (%) | 56 | 15 (26.79) | 41 (73.21) | |
| Age (years); mean±SD | 32.89±4.16 | 33.41±4.37 | 32.65±4.16 | 0.55 |
| BMI (kg/m2); mean±SD | 22.90±3.06 | 23.52±3.14 | 22.68±3.07 | 0.37 |
| TSH (μU/mL); mean±SD | 4.60±1.77 | 5.22±1.26 | 4.82±1.90 | 0.66 |
| FH DM; n (%) | 23 (41.07) | 9 (16.07) | 14 (25.00) | 0.084 |
| Thyroid antibodies; n (%) | 21 (37.50) | 6 (10.71) | 15 (26.79) | 0.77 |
| Anti-TPO; n (%) | 19 (33.92) | 6 (10.71) | 13 (23.21) | 0.46 |
| Anti-TG; n (%) | 7 (12.50) | 2 (3.57) | 5 (8.93) | 0.91 |
FH DM: family history of diabetes mellitus; Anti-TG: thyroglobulin antibodies; Anti-TPO: thyroid peroxidase antibodies; BMI: body mass index.
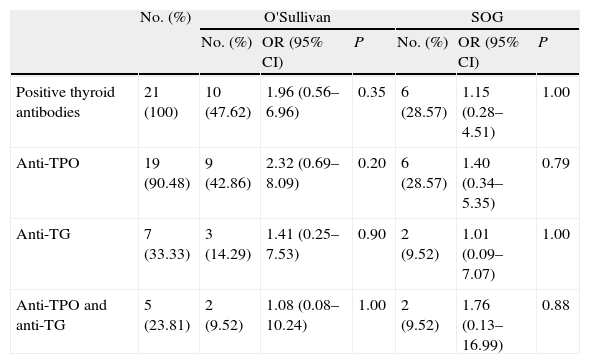
The potential impact of thyroid autoimmunity (in patients with TSH above the goal at the start of pregnancy) on GD development was analyzed. Ten of the 21 patients with thyroid autoimmunity (47.62%) had a positive O'Sullivan test. Six of these 10 patients (28.57%) were finally diagnosed with GD. Among the 35 pregnant women with no autoimmunity, 11 (31.43%) had a positive O'Sullivan test, and 9 of them (25.71%) were diagnosed with GD. Based on autoimmunity results, patients were divided into three groups by antibody type to analyze the risk of GD development depending on the elevated antibody. A logistic regression analysis found no significant differences between patients diagnosed with GD positive for TPO and/or TG antibodies and patients with no autoimmunity (Table 2).
Prevalence and risk of positive O'Sullivan test and gestational diabetes in the presence of thyroid autoimmunity, classified by type of thyroid antibodies.
| No. (%) | O'Sullivan | SOG | |||||
| No. (%) | OR (95% CI) | P | No. (%) | OR (95% CI) | P | ||
| Positive thyroid antibodies | 21 (100) | 10 (47.62) | 1.96 (0.56–6.96) | 0.35 | 6 (28.57) | 1.15 (0.28–4.51) | 1.00 |
| Anti-TPO | 19 (90.48) | 9 (42.86) | 2.32 (0.69–8.09) | 0.20 | 6 (28.57) | 1.40 (0.34–5.35) | 0.79 |
| Anti-TG | 7 (33.33) | 3 (14.29) | 1.41 (0.25–7.53) | 0.90 | 2 (9.52) | 1.01 (0.09–7.07) | 1.00 |
| Anti-TPO and anti-TG | 5 (23.81) | 2 (9.52) | 1.08 (0.08–10.24) | 1.00 | 2 (9.52) | 1.76 (0.13–16.99) | 0.88 |
Anti-TG: thyroglobulin antibodies; Anti-TPO: thyroid peroxidase antibodies; 95% CI: 95% confidence interval; OR: odds ratio; OGTT: oral glucose tolerance test.
Data were obtained by exact logistic regression using LogXact-8 Cytel software.
This study showed a high prevalence of GD in pregnant women with TSH levels above the optimal range recommended by the ATA.20 In our series, however, no relationship was seen between GD and thyroid autoimmunity in pregnancy.
As noted above, this study was intended to analyze whether the presence of thyroid autoimmunity in patients with TSH levels above the reference range could increase the risk of GD development. This assumption was based on evidence linking inflammation to both insulin resistance7 and thyroid autoimmunity.11 Thus, a study showed decreased glucose tolerance and a trend to increased circulating fasting insulin levels at the end of pregnancy in women with thyroid antibodies who subsequently experienced post-partum thyroiditis, which was not attributable to islet cell autoimmunity.22 However, the currently available reference literature on this issue is limited, and results are controversial.23–28
In our population of pregnant women, monitored during pregnancy because they had TSH levels above the goal established at the start of pregnancy (goal<2.5μU/mL), a higher GD prevalence was not found in women with positive TPO and/or TG antibodies. However, GD prevalence in our sample was clearly higher than documented in the general Spanish population, which is approximately 10%.3–6
When factors identified as independent predictors for GD (including age, BMI, and family history of diabetes mellitus)29,30 were analyzed in our series, no significant differences were found between pregnant women diagnosed with GD and the control group. No differences were also seen between the groups with regard to the TSH level at the start of pregnancy or the thyroid antibody titer. It should be noted, however, that pregnant women diagnosed with GD had higher mean TSH levels as compared to the control group, a circumstance which may be investigated in studies on larger samples. There was also a greater prevalence of TPO as compared to TG antibodies, with a higher GD rate in this group, despite the fact that no statistical significance was found in the regression analysis, possibly because of the small sample size. These results are similar to those previously reported.24
Limitations of our study included its retrospective nature and the small sample size. It should also be recognized that it would have been interesting to test glutamic acid decarboxylase (GAD-65) antibodies, and to have a control group of pregnant women with TSH levels within the established goal during pregnancy (<2.5μU/mL). However, this was a retrospective study in standard clinical practice, in which thyroid antibodies are not routinely tested in the absence of thyroid dysfunction or a relevant history that warrants such testing.
In conclusion, presence of thyroid autoimmunity in patients with TSH levels above the desired range at the start of pregnancy does not predict for GD development. However, GD prevalence in our sample was higher than reported in the general Spanish population, which suggests the need for closer monitoring in pregnant women with TSH≥2.5μU/mL.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Pascual Corrales E, Andrada P, Aubá M, Ruiz Zambrana Á, Guillén Grima F, Salvador J, et al. ¿Existe mayor riesgo de diabetes gestacional en pacientes con disfunción tiroidea autoinmune? Endocrinol Nutr. 2014;61:377–381.