To assess whether levothyroxine treatment improves functional capacity in patients with chronic heart failure (New York Heart Association class I–III) and subclinical hypothyroidism.
MethodsOne hundred and sixty-three outpatients with stable chronic heart failure followed up for at least 6 months were enrolled. A physical examination was performed, and laboratory tests including thyroid hormone levels, Doppler echocardiogram, radionuclide ventriculography, and Holter monitoring were requested. Functional capacity was assessed by of the 6-min walk test. Patients with subclinical hypothyroidism were detected and, after undergoing the s6-min walk test, were given replacement therapy. When they reached normal thyrotropin (TSH) levels, the 6-min walk test was performed again. The distance walked in both tests was recorded, and the difference in meters covered by each patient was analyzed.
ResultsPrevalence of subclinical hypothyroidism in patients with heart failure was 13%. These patients walked 292±63m while they were hypothyroid and 350±76m when TSH levels returned to normal, a difference of 58±11m (P<.011). Patients with normal baseline TSH levels showed no significant difference between the two 6-min walk tests.
ConclusionsPatients with chronic heart failure and subclinical hypothyroidism significantly improved their physical performance when normal TSH levels were reached.
Evaluar si el tratamiento con levotiroxina mejora la capacidad funcional en pacientes con insuficiencia cardíaca crónica clase funcional i-iii de la New York Heart Association e hipotiroidismo subclínico.
MétodosSe incluyeron 163 pacientes ambulatorios con insuficiencia cardíaca crónica estable y con un mínimo de seguimiento de 6 meses. Se realizó un examen clínico y se solicitaron pruebas de laboratorio que incluyeron hormonas tiroideas, ecocardiograma con doppler, ventriculografía radioisotópica y un estudio Holter. La capacidad funcional se evaluó por medio de una caminata de 6min. Se detectaron los pacientes con hipotiroidismo subclínico que recibieron tratamiento sustitutivo y, una vez con valores normales de tirotropina (TSH), se les realizó una nueva caminata de 6min. Se registraron los metros recorridos en cada prueba y se analizó la diferencia de los metros caminados en cada paciente.
ResultadosObservamos una prevalencia de hipotiroidismo subclínico del 13% en pacientes con insuficiencia cardíaca. Mientras se encontraban hipotiroideos, los metros recorridos fueron de 292±63, y una vez alcanzados valores normales de TSH, de 350±76. La diferencia en metros fue de 58±11 (p<0,011). Los pacientes con valores normales de TSH no mostraron diferencias significativas entre las 2 pruebas.
ConclusionesLos pacientes con insuficiencia cardíaca crónica e hipotiroidismo subclínico, una vez eutiroideos, mejoraron de forma significativa su rendimiento físico.
Heart failure (HF) syndrome is highly complex and starts with impaired ventricular, diastolic and/or systolic function, but subsequently causes and involves biochemical, metabolic, hormonal, and neurohormonal changes.1 The relationship between thyroid hormones, the heart, and the peripheral vascular system is well known. Thyroid hormones have relevant actions upon the heart and circulation, inducing multiple changes including hemodynamic changes and effects on cardiac cells mediated through gene expression.2–4
Subclinical hypothyroidism (SH) is a common biochemical condition defined as thyroid-releasing hormone (TSH) elevation with circulating thyroid hormone levels within the normal reference range.5 No international consensus exists yet about the cut-off point from which TSH levels should be considered as pathological. The US National Health and Nutrition Examination Survey III assessed a sample of apparently healthy and euthyroid subjects with negative anti-thyroid antibodies. In this population median TSH levels were 1.39μIU/L, with values for 95% of the samples ranging from 0.45 to 4.12μIU/L.6 These values were taken as a reference to establish the normal cut-off point for TSH in a consensus for the diagnosis and treatment of SH.7 However, the US National Academy of Clinical Biochemistry suggested as normal reference limits values ranging from 0.4 to 2.5μIU/L,5 while the American Association of Clinical Endocrinologists established a range from 0.3 to 3.0μIU/L.8
The prevalence of SH in the general population is 1–11%, and up to 20% in women over 60 years.6,9–12 The clinical relevance of SH has not been established yet. Patients with SH have increases in low density lipoprotein levels and a prevalence of coronary disease and peripheral artery disease.13,14 Thyroid changes in HF are associated with poorer functional class and more impaired ventricular function. Thus, in addition to conventional methods, thyroid function tests may be useful for assessing the course or prognosis of patients with HF.15 The use of hormone replacement therapy in patients with SH would therefore appear to be advisable, and regular thyroid profile assessment in HF would provide valuable information for the prognostic stratification of these patients.
Because of the existing controversy concerning the use of replacement therapy in this type of patient, the purpose of this study was to assess whether, in the subgroup of patients with HF and SH, hormone profile correction results in an improved functional capacity, as measured by the 6-min walk test.
Subjects and methodsThis was a prospective, open label, intervention study with subjects acting as their own controls, in which consecutive outpatients from two centers were enrolled. After agreeing to participate in the study, and provided they met the requirements for clinical studies of the research and teaching department of Hospital Militar Central, including the signature of an informed consent, patients with HF were enrolled if they had had at least six months of follow-up, had been stable for at least one month, and were receiving optimal doses–or maximal tolerated doses–of angiotensin II converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, and spironolactone.
The study inclusion criteria were as follows: age>21 years, left ventricular diastolic diameter>55mm, and ejection fraction<35%. The exclusion criteria were as follows: patients with surgery indicated for any reason, with cardiomyopathy of valvular and hypertrophic origin, being treated with amiodarone, with complex ventricular arrhythmia, with a history of sudden death and/or syncope of probable arrhythmic origin, with an implanted cardiac defibrillator, and with motor inability to perform the 6-min walk.
Each participant underwent a physical examination and an electrocardiogram, a two-dimensional Doppler echocardiogram, radioisotopic ventriculography, and 24-h Holter monitoring were performed. Patients with ischemic etiology also underwent a myocardial perfusion study. The laboratory blood tests requested included the measurement of TSH, triiodothyronine, and free thyroxine by electrochemiluminescence immunoassay. Reference ranges were 0.27–4.2μIU/L, 0.8–2.0ng/mL for triiodothyronine, and 0.7–1.7ng/dL for free thyroxine.
SH was defined as TSH elevation in two consecutive laboratory measurements with levels above the 95th percentile according to the National Health and Nutrition Examination Survey III (4.12μIU/L), with levels of circulating thyroid hormones within the normal range. Functional capacity was assessed using the 6-min walk test. Once enrolled, patients with SH were detected, and the condition was confirmed by a second biochemical measurement at two months. All patients performed the first 6-min test. After a 15min rest, sitting blood pressure using a calibrated sphygmomanometer and heart rate were measured by the same operator. The patients were subsequently instructed to walk for 6min at the fastest possible speed along a 30m corridor. Once the test was completed, the same variables were controlled.
Patients with HF and SH were referred to the endocrinology department to be assessed by an endocrinologist, who prescribed replacement therapy for SH. Patients without SH were appointed for a second 6-min walk test 20 days after the first, while those treated for SH were appointed for the second test 30 days after they were shown to have returned to normal TSH levels. The meters walked in each test were recorded, and the difference in meters walked was analyzed in each patient. As a safety measurement, all participants in the SH group were given a new 24-h Holter after receiving hormone replacement therapy for 30 days.
Statistical analysisParametric continuous variables were expressed as mean±standard deviation or as median±interquartile range, and non-parametric variables as ratios or percentages as appropriate.
For continuous variables, after testing the normality of sample distributions using a Shapiro–Wilk test and eventually attempting normalization using the corresponding transformation (logarithmic transformation), within-group comparisons were made using a Student's t-test for paired data, and between-group comparisons were made using a Student's t-test for parallel samples. In the event of clearly non-normal distributions, non-parametric methods were used for inferential statistical analysis (test of proportions).
A two-sided value of p≥0.05 was considered statistically significant. According to laboratory sample data, the number of patients to be included for a change in reference values of 20% or more to be detected in the walk with treatment, with an error alpha<0.05 and a power of ≥90%, was 20 subjects.
ResultsThe total HF group consisted of 163 patients, of whom 13% had SH (95% CI 7.8–18.2%).
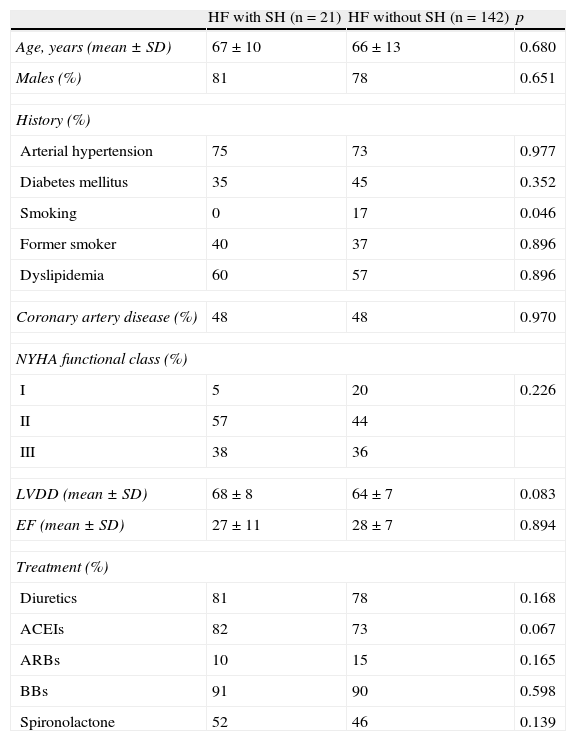
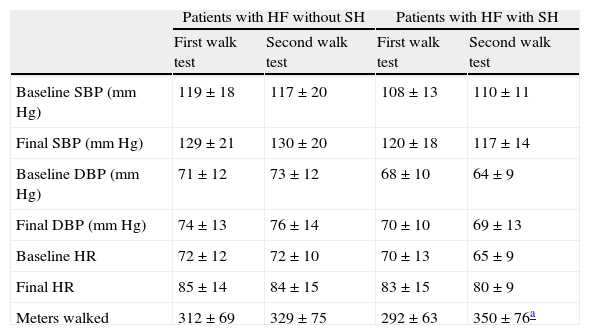
Table 1 shows the characteristics of HF subgroups without SH (n=142) and with SH (n=21). As shown in Table 2, there were no significant differences in heart rate or systolic and diastolic blood pressure between the first and second walk tests in any of the two groups. No significant differences in these variables were found either between both groups in each walk test. The HF group showed a significant increase in meters walked in the second test (58m; p<0.011), while the group without SH experienced a nonsignificant increase in walked distance of less than one-third (17m; p=0.20).
Clinical characteristics of patients with heart failure with and without subclinical hypothyroidism.
| HF with SH (n=21) | HF without SH (n=142) | p | |
| Age, years (mean±SD) | 67±10 | 66±13 | 0.680 |
| Males (%) | 81 | 78 | 0.651 |
| History (%) | |||
| Arterial hypertension | 75 | 73 | 0.977 |
| Diabetes mellitus | 35 | 45 | 0.352 |
| Smoking | 0 | 17 | 0.046 |
| Former smoker | 40 | 37 | 0.896 |
| Dyslipidemia | 60 | 57 | 0.896 |
| Coronary artery disease (%) | 48 | 48 | 0.970 |
| NYHA functional class (%) | |||
| I | 5 | 20 | 0.226 |
| II | 57 | 44 | |
| III | 38 | 36 | |
| LVDD (mean±SD) | 68±8 | 64±7 | 0.083 |
| EF (mean±SD) | 27±11 | 28±7 | 0.894 |
| Treatment (%) | |||
| Diuretics | 81 | 78 | 0.168 |
| ACEIs | 82 | 73 | 0.067 |
| ARBs | 10 | 15 | 0.165 |
| BBs | 91 | 90 | 0.598 |
| Spironolactone | 52 | 46 | 0.139 |
ARBs: angiotensin receptor blockers; BBs: beta-blockers; LVDD: left ventricular diastolic diameter; SD: standard deviation; EF: ejection fraction; SH: subclinical hypothyroidism; HF: heart failure; ACEIs: angiotensin-converting enzyme inhibitors; NYHA: New York Heart Association.
Characteristics of variables analyzed in the two groups in both 6-min walk tests.
| Patients with HF without SH | Patients with HF with SH | |||
| First walk test | Second walk test | First walk test | Second walk test | |
| Baseline SBP (mmHg) | 119±18 | 117±20 | 108±13 | 110±11 |
| Final SBP (mmHg) | 129±21 | 130±20 | 120±18 | 117±14 |
| Baseline DBP (mmHg) | 71±12 | 73±12 | 68±10 | 64±9 |
| Final DBP (mmHg) | 74±13 | 76±14 | 70±10 | 69±13 |
| Baseline HR | 72±12 | 72±10 | 70±13 | 65±9 |
| Final HR | 85±14 | 84±15 | 83±15 | 80±9 |
| Meters walked | 312±69 | 329±75 | 292±63 | 350±76a |
HR: heart rate; SH: subclinical hypothyroidism; HF: heart failure; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Median TSH levels in the SH subgroup (21 patients) were 6.25μIU/L (5.12–14.46) at baseline, and 2.53μIU/L (2.03–3.69) after treatment with levothyroxine (p<0.0001). The median time required by patients with SH to normalize TSH levels was 36 days (minimum 28 days, maximum 45 days). In this group, the second walk test was performed after a median of 43 days (minimum 35 days, maximum 52 days), the mean levothyroxine dose used was 86.90±32.22mcg/day (median of 75mcg/day, interquartile range 75–100), and there were no side effects of replacement therapy.
DiscussionThyroid function changes are very common in patients with HF. Interest in the role of thyroid hormones in HF has been revived in recent years due to the awareness of their effects on cardiac contractility and relaxation, experimental findings strongly supporting the hypothesis that thyroid hormone functions are important for the preservation of cardiac anatomy and function under physiological and abnormal conditions and, also, because of evidence that minimal changes in thyroid hormone function are associated with poorer prognosis.16–20 A significant number of patients with HF are often seen to also have SH. Our study was therefore aimed at verifying what happened to the functional capacity of these patients if the biochemical change was corrected, and a standard levothyroxine dose was not decided on because the objective was euthyroidism regardless of the dose. In patients with HF and SH, levothyroxine treatment was shown to significantly increase functional capacity in meters in the 6-min walk test as compared to a group of patients with HF but not with SH. Although an improved performance may be expected when the test is done more than once, due to patient familiarity with the test, as is shown in Table 2, the increase seen in the group with HF but not with SH was less than one-third than that experienced by patients with both HF and SH (17 vs 58m). The decrease in systemic vascular resistance seen after thyroid hormone administration may be of help in improving cardiac function without any cost in oxygen consumption, because it acts by decreasing after load due to a vasodilating effect. In addition, this reduction in systemic vascular resistance decreases diastolic blood pressure, thus increasing minute volume. This increase in minute volume supports increases in the basal metabolic index and oxygen consumption, which increases oxygen release to the periphery. These findings may be explained by blood flow redistribution to the skeletal muscles through vasodilation, or by muscle metabolism improvement due to the local action of T4, which stimulates protein synthesis, mitochondrial enzymes, and the conversion of fast fibers to slow fibers, a similar situation to that which occurs in physical training.12,21
Several studies have investigated the impact of SH on patients with HF. However, conflicting results have often been found. For example, Rodondi et al. reported a four-year follow-up of patients with SH in whom an increase was seen in events associated with HF with levels of 7μIU/L or higher as compared to euthyroid participants.22 Silva-Tinoco et al. in a prospective study enrolling euthyroid HF patients who subsequently developed SH, reported a poor prognosis and a higher hospitalization rate as compared to patients who remained euthyroid.23 By contrast, Frey et al. recently showed, in a cohort with a mean follow-up of three years, that SH does not involve a poorer evolutive prognosis or a greater mortality risk.24
Replacement therapy with thyroid hormones in HF has been assessed in studies with small sample sizes, but there is a strong argument favoring treatment because genetic expression defects in patients with HF are similar to those found in patients with hypothyroidism, and changes are reversible with replacement therapy.21,25–29
It has been postulated that thyroid changes are an adaptive or maladaptive mechanism to the increased catabolism operating in patients with advanced HF, but the role of SH is currently unknown. Regardless of considerations as to whether this may be an adaptive or a maladaptive mechanism or, eventually, a comorbidity, what is clear is that the proportion of patients with SH is not negligible, and although this is by definition an asymptomatic biochemical change, daily practice shows that this is not true and, according to the literature, from 25% to 50% of patients with SH experience some symptoms explained by this disorder.
Our study showed a 13% prevalence of SH (95% CI 7.8–18.2%), with a median TSH level of 6.25μIU/L, in a mostly male population, and although the method used in the study was not ideal for assessing the response to levothyroxine treatment, an improvement was seen in the distance walked after a euthyroid state was achieved. Good patient control was seen, and replacement therapy was not associated with relevant side effects. Treatment was also associated with a significant improvement in functional capacity. We therefore think it appropriate to routinely measure thyroid hormones in this group of patients.
This study had some limitations. While the sample was small, the proportion of patients with SH was similar to that in prior reports. This study was not randomized, and was therefore not ideal for assessing the response to treatment. A design using subjects as their own controls was decided upon because this type of study requires a smaller sample, and since the patients were to be recruited in only two centers and within a maximum of two years, we thought this would be the most appropriate design for the study objectives. The open label nature of a study may make it difficult to conduct, but this risk had to be assumed to take advantage of the relatively limited number of cases and not to deprive some patients of treatment.
The 6-min walk test is a controversial method because some studies in which it has been used, as well as its results, have been heterogeneous in terms of the characteristics of the study population, the ongoing treatment, and the test procedure used. Despite this, it has been used at the heart failure section of our hospital for more than 15 years as a routine supplemental method in patients with HF. The 6-min walk test is an easy, safe, and inexpensive method. Test subjectivity may be minimized by being performed by the same examiner and by stimulating patients during the test, which makes it quite reliable. Thus, many prior large studies have used it as a procedure to assess functional capacity in this type of patient, and many such studies have shown the test to better correlate to ergometry as compared to oxygen consumption with regard to the occurrence of future events.30
We conclude that HF patients with SH treated with levothyroxine significantly improve their physical performance, as measured by the 6-min walk test, and that this warrants routine thyroid hormone screening, especially if these optimally treated patients do not improve their functional capacity.
Conflicts of interestThe authors state that they have no conflicts of interest.
The authors especially thank Mrs. Eleonora Vanasco and Mrs. Alicia Aubry for manuscript correction.
Please cite this article as: Curotto Grasiosi J, Peressotti B, Machado RA, Filipini EC, Angel A, Delgado J, et al. Mejoría de la capacidad funcional tras tratamiento con levotiroxina en pacientes con insuficiencia cardíaca crónica e hipotiroidismo subclínico. Endocrinol Nutr. 2013;60:427–432.