MODY (maturity onset diabetes of the young) is a group of well-defined diseases clinically characterised by onset before 25 years of age that does not require insulin treatment (at least initially) to prevent the formation of ketone bodies and autosomal dominant inheritance. Despite the importance of accurate classification, it is not always simple to catalogue the diagnosis of a young patient with diabetes, and genetic studies are often improperly used.
MethodsWe describe the clinical features of patients negative for MODY2 and MODY3 and compared them to patients positive for these subtypes.
ResultsAll patients with MODY3 had been diagnosed before 25 years of age and required drug therapy for blood glucose control. MODY2 patients were diagnosed at the first laboratory workup either incidentally or as part of gestational diabetes screening. The clinical description of the 19 patients negative for MODY2 and MODY3 showed that only two patients presented a clinical picture consistent with MODY3 and one patient with MODY2.
ConclusionsClinical features can be used for early exclusion of a MODY2 or MODY3 diagnosis and may reduce the need for genetic testing.
La diabetes tipo MODY (del inglés maturity onset diabetes of the young) constituye un grupo de patologías bien definidas y caracterizadas por su aparición antes de los 25 años, herencia autosómica dominante y por el hecho de que no precisan un tratamiento con insulina (al menos, inicialmente) para evitar la formación de cuerpos cetónicos. A pesar de la importancia de una clasificación precisa del paciente diabético, no siempre resulta sencillo clasificar el diagnóstico de un paciente joven con diabetes, y los estudios genéticos, a menudo, se usan de forma inadecuada.
MétodosSe describen las características clínicas de pacientes cuyo estudio para MODY2 y MODY3 resultó negativo, y se comparan con las características de pacientes con resultado de estudio positivo.
ResultadosTodos los pacientes con MODY3 habían sido diagnosticados antes de los 25 años de edad y requerían algún tratamiento farmacológico para controlar la glucemia. Los pacientes con MODY2 fueron diagnosticados a partir de la primera analítica realizada, bien de forma accidental o dentro de un contexto de cribado de diabetes gestacional. La descripción clínica de los 19 pacientes cuyo estudio para MODY2 y MODY3 resultó negativo, mostró que sólo dos pacientes presentaban un cuadro clínico compatible con MODY3 y solo un paciente con MODY2.
ConclusionesLas características clínicas pueden ser utilizadas para excluir el diagnóstico de MODY2 y MODY3, y ello puede reducir la necesidad de estudios genéticos.
The term MODY (maturity onset diabetes of the young) has traditionally been used to describe forms of diabetes mellitus that usually presents before 25 years of age and do not require insulin to prevent ketone body formation.1,2 These heterogeneous group of diseases are part of monogenic diabetes, caused by mutations in the genes involved in pancreatic beta cell function; nine types have been described to date, all characterised by autosomal dominant inheritance and have a well-defined clinical presentation, risk of associated complications and response to different treatments.3
Except for MODY2 or GCK-monogenic diabetes (Online Mendelian Inheritance in Man ID #125851), which results from an inactivating mutation in the glucokinase gene,4,5 all MODY subtypes are caused by mutations in genes encoding different transcription factors: MODY1 (#125850) due to HNF4A mutations,6 MODY3 or HNF1A-monogenic diabetes (#600496) to HNF1A mutations,7 MODY4 (#606392) to IPF1 mutations,8 MODY5 (#137920) to HNF1B mutations,9 MODY6 (#606394) to NEUROD1 mutations,10 MODY7 (#610508) to KLF11 mutations,11 MODY8 (#609812) to CEL VNTR12 and MODY9 (#612225) to PAX4 mutations.13
Although the actual prevalence of the condition is unknown, it is estimated to account for 1–5% of all forms of diabetes.3 In Spain MODY2 and MODY3 diabetes are the most common forms,14–19 and only one family with MODY516 diabetes and two families with MODY1 diabetes15,19 have been described.
According to our experience and that of other groups, MODY studies are negative in 16–45% of patients tested.20 Nevertheless, recent publications from Spanish groups report much higher figures (69.7%18 and 74.5%),19 thus raising the possibility of mutations not yet described (designated MODY-X). To our knowledge, however, no publication describes the clinical features of patients with negative results or the extent to which these features match those usually seen in MODY.
The purpose of our study was to describe the clinical features of patients analysed by our group who were negative for MODY2 and MODY3 and to compare them to patients positive for these subtypes.
MethodsWe conducted a retrospective review of the medical records of 52 patients assessed at the various outpatient clinics of the Endocrinology and Nutrition Department at the Hospital and University Complex in Albacete, Spain, who underwent a genetic study for MODY2 and MODY3. The reviews did not include MODY2 and MODY3 subjects who were known relatives of an index case or for whom clinical information was insufficient. Once written informed consent was obtained, the genetic study was carried out at the Human Genetics Laboratory (School of Medicine of Albacete, University of Castilla-La Mancha) after excluding pancreatic autoimmunity (serum glutamate decarboxylase antibodies by immunoradiometric assay<0.90U/ml), automatic DNA sequencing of the ten coding exons (1a, 2–10) and promoter region (−1 to −870) of GCK (MODY2) as well as the ten coding exons and promoter region (−1 to −291) of HNF1A (MODY3).
The following information was recorded for all patients: sex, age at diagnosis, familial history of diabetes, reason for diagnosis (e.g. incidental diagnosis in a laboratory workup for another reason, laboratory workup for clinical suspicion of diabetes, gestational diabetes), diagnosis of hypertension (blood pressure≥130/80mmHg on repeated occasions or need for antihypertensive medication), current treatment prescribed by the endocrinologist (e.g. diet and exercise, oral hypoglycaemic agents, insulin and oral hypoglycaemic agents, or insulin alone), BMI (body mass index), DCCT-aligned glycated haemoglobin (HbA1c; determined by HPLC), HDL (high density lipoprotein)-cholesterol and triglycerides (by colorimetric enzymatic assay).
A descriptive analysis of the data was performed; all quantitative variables were expressed as the mean and standard deviation (SD) and all qualitative variables as percentages. The Kruskal–Wallis non-parametric test was used to compare the means. The χ2-test was used to compare proportions. SPSS 14.0 for Windows was used for the statistical analysis.
ResultsA total of 52 medical histories were reviewed: 11 (21.15%) patients from 7 families were positive for MODY3; 22 (42.30%) from 12 families were positive for MODY2, and 19 (36.53%) were negative for both MODY2 and MODY3. The analysis excluded one patient with MODY3 diagnosed during a family study, four patients with MODY2 (two diagnosed in a family study and two lost to follow-up) and one patient negative for MODY2 and MODY3 lost to follow-up.
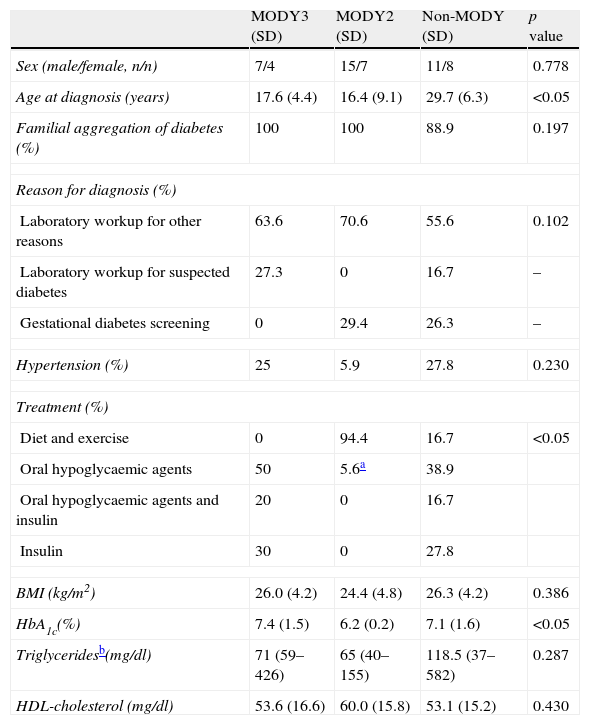
The clinical characteristics of each group are listed in Table 1. Statistically significant differences were observed between the groups in terms of age at diagnosis, treatment and HbA1c. All subjects with MODY3 had been diagnosed before 25 years of age and required drug therapy for blood glucose control. MODY2 patients were diagnosed at the first laboratory workup (age≥25 years in seven patients) either incidentally or during gestational diabetes screening. Only one patient required drug therapy for blood glucose control (46-year-old patient with a BMI of 32kg/m2, diagnosed with type 2 diabetes mellitus under metformin therapy, who underwent a genetic study for MODY2 after two of her children were diagnosed with MODY2). None of the three groups presented statistically significant differences according to sex, family history, reasons for diagnosis, high blood pressure, BMI, triglyceride or HDL-cholesterol levels.
Clinical characteristics of diabetic patients undergoing MODY genetic testing.
| MODY3 (SD) | MODY2 (SD) | Non-MODY (SD) | p value | |
| Sex (male/female, n/n) | 7/4 | 15/7 | 11/8 | 0.778 |
| Age at diagnosis (years) | 17.6 (4.4) | 16.4 (9.1) | 29.7 (6.3) | <0.05 |
| Familial aggregation of diabetes (%) | 100 | 100 | 88.9 | 0.197 |
| Reason for diagnosis (%) | ||||
| Laboratory workup for other reasons | 63.6 | 70.6 | 55.6 | 0.102 |
| Laboratory workup for suspected diabetes | 27.3 | 0 | 16.7 | – |
| Gestational diabetes screening | 0 | 29.4 | 26.3 | – |
| Hypertension (%) | 25 | 5.9 | 27.8 | 0.230 |
| Treatment (%) | ||||
| Diet and exercise | 0 | 94.4 | 16.7 | <0.05 |
| Oral hypoglycaemic agents | 50 | 5.6a | 38.9 | |
| Oral hypoglycaemic agents and insulin | 20 | 0 | 16.7 | |
| Insulin | 30 | 0 | 27.8 | |
| BMI (kg/m2) | 26.0 (4.2) | 24.4 (4.8) | 26.3 (4.2) | 0.386 |
| HbA1c(%) | 7.4 (1.5) | 6.2 (0.2) | 7.1 (1.6) | <0.05 |
| Triglyceridesb(mg/dl) | 71 (59–426) | 65 (40–155) | 118.5 (37–582) | 0.287 |
| HDL-cholesterol (mg/dl) | 53.6 (16.6) | 60.0 (15.8) | 53.1 (15.2) | 0.430 |
SD: standard deviation.
Values are mean (SD) unless stated otherwise.
BMI: body mass index; HbA1c: glycated haemoglobin; HDL: high density lipoprotein.
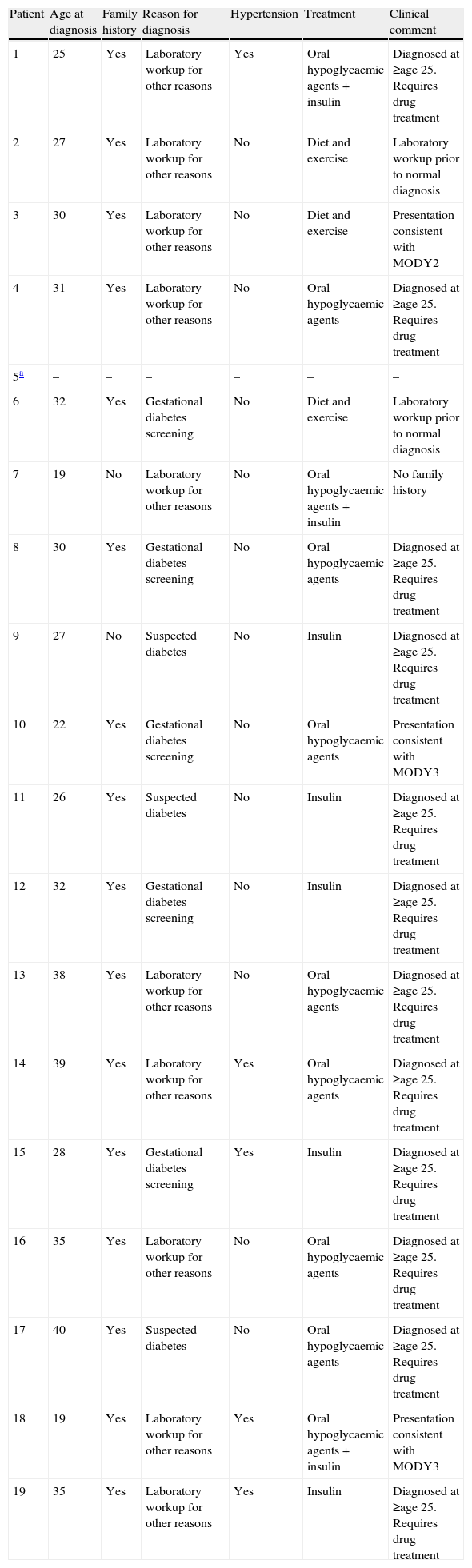
Table 2 shows the clinical features for the 19 patients negative for MODY2 and MODY3, as well as clinical comments in favour or against a “clinical” diagnosis of MODY2 and MODY3. The clinical description of the 19 patients negative for MODY2 and MODY3 showed that only two patients presented a clinical picture consistent with MODY3 (diagnosis <25 years, negative pancreatic islet autoimmunity, positive family history, no clinical evidence of type 2 diabetes, need for drug therapy to control glycaemia) and one patient with a clinical picture consistent with MODY2 (diagnosis at 30 years of age during first routine laboratory workup, no need for drug therapy to control blood glucose, negative pancreatic islet autoimmunity, family history of diabetes, no clinical evidence of type 2 diabetes).
Clinical features of the 19 patients negative for MODY2 and MODY3.
| Patient | Age at diagnosis | Family history | Reason for diagnosis | Hypertension | Treatment | Clinical comment |
| 1 | 25 | Yes | Laboratory workup for other reasons | Yes | Oral hypoglycaemic agents+insulin | Diagnosed at ≥age 25. Requires drug treatment |
| 2 | 27 | Yes | Laboratory workup for other reasons | No | Diet and exercise | Laboratory workup prior to normal diagnosis |
| 3 | 30 | Yes | Laboratory workup for other reasons | No | Diet and exercise | Presentation consistent with MODY2 |
| 4 | 31 | Yes | Laboratory workup for other reasons | No | Oral hypoglycaemic agents | Diagnosed at ≥age 25. Requires drug treatment |
| 5a | – | – | – | – | – | – |
| 6 | 32 | Yes | Gestational diabetes screening | No | Diet and exercise | Laboratory workup prior to normal diagnosis |
| 7 | 19 | No | Laboratory workup for other reasons | No | Oral hypoglycaemic agents+insulin | No family history |
| 8 | 30 | Yes | Gestational diabetes screening | No | Oral hypoglycaemic agents | Diagnosed at ≥age 25. Requires drug treatment |
| 9 | 27 | No | Suspected diabetes | No | Insulin | Diagnosed at ≥age 25. Requires drug treatment |
| 10 | 22 | Yes | Gestational diabetes screening | No | Oral hypoglycaemic agents | Presentation consistent with MODY3 |
| 11 | 26 | Yes | Suspected diabetes | No | Insulin | Diagnosed at ≥age 25. Requires drug treatment |
| 12 | 32 | Yes | Gestational diabetes screening | No | Insulin | Diagnosed at ≥age 25. Requires drug treatment |
| 13 | 38 | Yes | Laboratory workup for other reasons | No | Oral hypoglycaemic agents | Diagnosed at ≥age 25. Requires drug treatment |
| 14 | 39 | Yes | Laboratory workup for other reasons | Yes | Oral hypoglycaemic agents | Diagnosed at ≥age 25. Requires drug treatment |
| 15 | 28 | Yes | Gestational diabetes screening | Yes | Insulin | Diagnosed at ≥age 25. Requires drug treatment |
| 16 | 35 | Yes | Laboratory workup for other reasons | No | Oral hypoglycaemic agents | Diagnosed at ≥age 25. Requires drug treatment |
| 17 | 40 | Yes | Suspected diabetes | No | Oral hypoglycaemic agents | Diagnosed at ≥age 25. Requires drug treatment |
| 18 | 19 | Yes | Laboratory workup for other reasons | Yes | Oral hypoglycaemic agents+insulin | Presentation consistent with MODY3 |
| 19 | 35 | Yes | Laboratory workup for other reasons | Yes | Insulin | Diagnosed at ≥age 25. Requires drug treatment |
In 1997, an international expert committee analysed the cumulative knowledge at the time and recommended a new diabetes classification based on pathogenesis and aetiology; the classification was later backed by the American Diabetes Association and the World Health Organization (WHO). The third group of this classification includes genetic defects that affect beta cell function, and among them MODY.
Although important, the diagnostic classification of a young diabetic subject is not always simple because the onset of type 1 diabetes still accounts for a high percentage of all diagnoses during the middle years of life,21 because the increased prevalence of obesity is causing an increase in the incidence of type 2 diabetes at increasingly younger ages,22 and because there is a greater capacity for the aetiological diagnosis of specific forms of diabetes associated with genetic defects.
As classification is difficult, clinicians may request monogenic diabetes genetic studies for subjects who present atypical clinical features for type 1 and type 2 diabetes mellitus but do not meet the clinical characteristics of MODY. This could lead to an increase in negative results with the consequential increase in health costs, and may cause some confusion in the clinician about the diagnosis, possibly resulting in a MODY-X diagnosis in patients without the clinical features of MODY and with negative studies.
Our study confirmed a higher frequency of MODY2 (22 patients from 12 families) compared to MODY3 (11 patients from 7 families) as well as a high percentage of negative results (36.5%). Confirmation of the specific clinical characteristics of MODY2 and MODY3 is also of interest. All patients positive for MODY2 presented negative autoimmunity to pancreatic islets and had a family history of diabetes with similar clinical characteristics and, as described above, presented mild hyperglycaemia in the first laboratory workup. Therefore, a workup before the age of diagnosis showing normal blood glucose levels allowed MODY2 diagnosis to be excluded. Because MODY2 patients do not require drug therapy to control blood glucose, the need for drug therapy also excludes a MODY2 diagnosis. In our series, only one patient required drug therapy for blood glucose control but she was diagnosed with type 2 diabetes mellitus under metformin therapy; she underwent a genetic study for MODY2 after two of her children were diagnosed with MODY2.
In the case of MODY3, all patients had negative autoimmunity to pancreatic islets and a family history of diabetes with the same characteristics; all were diagnosed before 25 years of age and required drug therapy to maintain blood glucose control. We can assume that patients not diagnosed during a routine laboratory workup usually develop clinical symptoms that lead to a suspicion of diabetes before this age. Therefore, a diabetes diagnosis after 25 years of age or the absence of a need for drug therapy to control glycaemia from this age should exclude a MODY3 diagnosis.
The clinical description of the 19 patients negative for MODY2 and MODY3 showed that the clinical picture was consistent with MODY3 in only two patients and with MODY2 in only one. In these patients, we recommend additional pancreatic islet autoimmunity studies and if negative, genetic tests for MODY1.
In conclusion, MODY2 and MODY3 genetic studies are readily available and performed more often, even in patients with clinical features that are atypical for type 1 or type 2 diabetes mellitus and inconsistent with MODY2 and MODY3. This situation leads to more negative results with the consequent increase in costs and may even yield confusing diagnoses.
Conflict of interestsThe authors declare that there is no conflict of interests.
To María Luisa Casas Oñate and Dolores Montoya Martínez, nurses of the endocrinology and nutrition outpatient clinic, for their assistance.
To the Castilla-La Mancha Foundation for Diabetes (FUCAMDI), for financial aid provided to the Laboratory of Human Genetics, School of Medicine of Albacete.
Please cite this article as: Pinés Corrales PJ, et al. Importance of clinical variables in the diagnosis of MODY2 and MODY3. Endocrinol Nutr. 2011;58:341–6.