Hypomagnesemia is an uncommon biochemical change in outpatients, but may be detected in up to 12% of hospitalized patients, in whom factors such as total and enteral nutrition, diuretic use, diarrhea, hypoalbuminemia, and nephrotoxic drugs (antibiotics, chemotherapeutic, etc.) play a significant role in its occurrence. Symptoms related to hypomagnesemia usually occur with serum magnesium levels lower than 1.2mg/dL. The condition is very frequently associated with the presence of hypocalcemia, hypokalemia, and metabolic alkalosis. Different authors have recently reported several cases of symptomatic hypomagnesemia associated with long-term use of proton pump inhibitors (PPIs).
A 39-year-old Caucasian male attended the emergency room reporting cramps in the hands and feet and numbness in the face for the previous 24h. He had experienced similar episodes during the previous 4 years, which he associated with abundant diarrheal stools. The patient had hypertension, dyslipidemia, and a hiatal hernia. 5 years before, he had also suffered a right hemispheric cerebral infarction of a cardioembolic origin from which he recovered without sequelae. Since then, he had been receiving the following treatment: acenocoumarol 3mg daily, acetylsalicylic acid 100mg daily, atenolol 25mg every 12h, amlodipine 5mg daily, losartan potassium 50mg daily, hydrochlorothiazide 25mg daily, simvastatin 20mg daily, and omeprazole 40mg daily. He had not taken alcohol, herbal products, laxatives, or nephrotoxic drugs.
Upon arrival at the emergency room he had blood pressure of 118/62mmHg, heart rate of 103bpm, was eupneic with a basal oxygen saturation of 97%, and had no fever. Muscle twitching was found in the quadriceps muscles, and carpopedal spasm in the hands and feet. Chvostek and Trosseau signs were negative. Pulmonary, cardiac, and abdominal examinations were all normal. Laboratory test results included: creatinine 1.01mg/dL, urea 26mg/dL, Na+ 142mM/L, Cl− 102mM/L, K+ 2.2mM/L (3.5–5.5mequiv./L), Ca++ 6.87mg/dL (8.7–10.3mg/dL), Mg++ 0.7mg/dL (1.40–2.40mg/dL), P+ 1.8mg/dL (2.7–4.5mg/dL). Venous blood gases included pH 7.45, pCO2 43mmHg, and HCO3− 29.9mequiv./L. An electrocardiogram showed sinus rhythm with no ST and/or QTc changes. Chest and abdominal X-rays were normal. The patient was admitted to the endocrinology ward and intravenous replacement therapy with Mg++, K+ and Ca++ was urgently started in accordance with the established recommendations (4 ampoules of Mg++ 12mequiv. in 1000mL of 5% glucose solution in 24h, a 20mequiv./L KCl solution at a maximum rate of 10mequiv./h, and a load of 200mg elemental Ca++ followed by infusion at 2mg/kg/h). Laboratory tests were performed every 3h for electrolyte adjustment. After a few hours of treatment, the electrolytes normalized and the symptoms were resolved. Oral supplements of Ca++, Mg++, and K+ continued to be administered.
In laboratory tests performed in the 2 years prior to admission there were no Mg++ or P+ values recorded, and hypocalcemia (7.4; 8.4; 8.5) and hypokalemia (3.5; 2.2; 3.3) were the only noteworthy findings.
Tests for renal tubular disease, thyroid function, bone metabolism (PTH 35.8pg/mL, vitamin D 25-OH 38.10ng/mL), and hyperaldosteronism were normal. The patient was discharged home with no symptoms 10 days after admission. The thiazide was discontinued, and treatment was continued with oral supplements of Mg++ (4.25mmol/day) and Ca++ (1000mg/day). Three months later, the patient returned to the endocrinology outpatient clinic for a scheduled visit with the same symptoms. This time, laboratory test results included K+ 3.5mM/L, total Ca++ 8.2mg/dL, P+ 2.8g/dL, and Mg++ 1.10mg/dL. Gastrointestinal study was completed with functional tests, computed tomography of the chest and abdomen, colonoscopy, oral panendoscopy, and gastrointestinal transit to rule out malabsorptive or paraneoplastic syndromes. The only change found in all of these tests was a positive lactose intolerance test. Despite oral supplements of Ca++ and Mg++, hypomagnesemia and hypocalcemia persisted until omeprazole was discontinued and ranitidine 150mg every 12h was started. Oral magnesium salts and a lactose-free diet were maintained upon discharge. After 3 weeks, tests showed normalization of the electrolyte parameters. Oral supplements were discontinued, and there were no subsequent decreases in serum levels of Mg++ and Ca++.
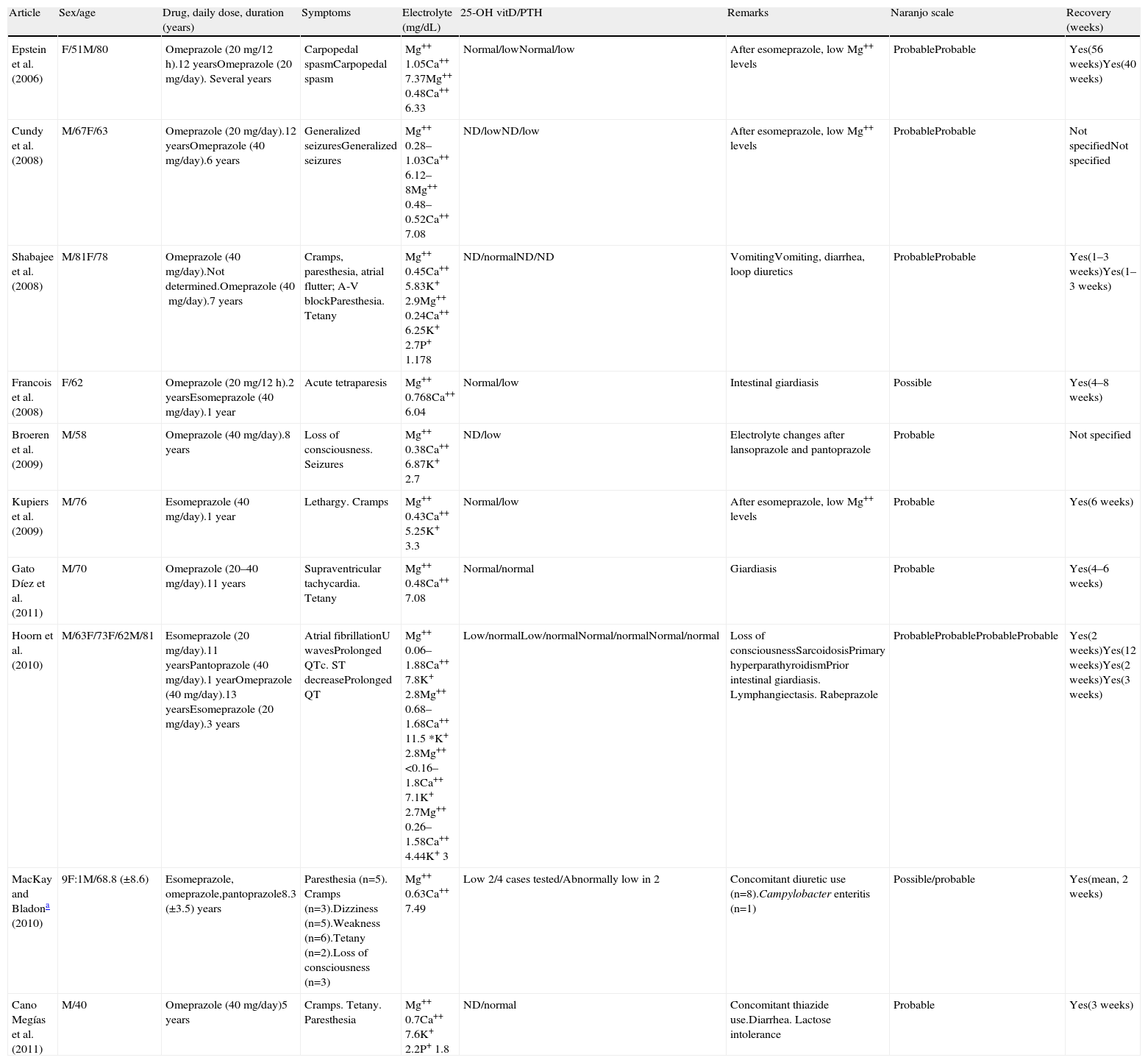
The exact incidence of this side effect of PPIs is currently unknown. A search in Pub Med/MEDLINE for recently published articles (2002–January 2011) using the terms hypomagnesemia, proton pump-inhibitor and/or omeprazole, pantoprazole, lansoprazole, found up to 25 published cases (including the one reported here). Table 1 summarizes the type of PPI, treatment dosage and duration, clinical characteristics, precipitating factors, therapeutic management, and prognosis in the reported cases. All patients had been treated with PPIs for at least 1 year (mean, 7.7 years; range, 1–13). There is a female sex predominance in the reported series (15 females, 10 males) which may be attributed to better treatment compliance.1 Mean age at occurrence of the condition was 67.9 years (range, 40–81 years). Hypomagnesemia is not only associated with omeprazole1 (n=15), but appears to be a drug-class side effect (esomeprazole,1–4 pantoprazole,1 lansoprazole,1,5 and rabeprazole4). In addition, the risk of hypomagnesemia is not dose-dependent. It may occur with both low or middle doses (20–40mg/day) and with 1 or 2 daily doses.2,6,7 Most patients (n=21, 84%) had neuromuscular signs such as weakness, dizziness, paresthesia,1 muscle cramps,8 carpopedal spasm,2 generalized seizures,2,5 and even loss of consciousness or acute tetraparesis.3 Three of these patients also had electrocardiographic changes including Supraventricular tachycardia,7 atrial flutter,8 and atrial fibrillation.4 Hoom et al.4 only reported cardiac rhythm disorders with no associated neuromuscular symptoms. Approximately one-fourth of all patients (n=6, 24% of the total) had no electrocardiographic changes. Triggering factors contributing to hypomagnesemia are frequently reported (Table 1). The triggering factors in the case reported here were chronic use of thiazide diuretics and diarrhea due to lactose intolerance, both of them known causes of hypomagnesemia from renal and gastrointestinal losses.
Summary of reported cases: clinical symptoms, electrolyte changes, treatment, and score in the Naranjo scale.
| Article | Sex/age | Drug, daily dose, duration (years) | Symptoms | Electrolyte (mg/dL) | 25-OH vitD/PTH | Remarks | Naranjo scale | Recovery (weeks) |
| Epstein et al. (2006) | F/51M/80 | Omeprazole (20mg/12h).12 yearsOmeprazole (20mg/day). Several years | Carpopedal spasmCarpopedal spasm | Mg++ 1.05Ca++ 7.37Mg++ 0.48Ca++ 6.33 | Normal/lowNormal/low | After esomeprazole, low Mg++ levels | ProbableProbable | Yes(56 weeks)Yes(40 weeks) |
| Cundy et al. (2008) | M/67F/63 | Omeprazole (20mg/day).12 yearsOmeprazole (40mg/day).6 years | Generalized seizuresGeneralized seizures | Mg++ 0.28–1.03Ca++ 6.12–8Mg++ 0.48–0.52Ca++ 7.08 | ND/lowND/low | After esomeprazole, low Mg++ levels | ProbableProbable | Not specifiedNot specified |
| Shabajee et al. (2008) | M/81F/78 | Omeprazole (40mg/day).Not determined.Omeprazole (40mg/day).7 years | Cramps, paresthesia, atrial flutter; A-V blockParesthesia. Tetany | Mg++ 0.45Ca++ 5.83K+ 2.9Mg++ 0.24Ca++ 6.25K+ 2.7P+ 1.178 | ND/normalND/ND | VomitingVomiting, diarrhea, loop diuretics | ProbableProbable | Yes(1–3 weeks)Yes(1–3 weeks) |
| Francois et al. (2008) | F/62 | Omeprazole (20mg/12h).2 yearsEsomeprazole (40mg/day).1 year | Acute tetraparesis | Mg++ 0.768Ca++ 6.04 | Normal/low | Intestinal giardiasis | Possible | Yes(4–8 weeks) |
| Broeren et al. (2009) | M/58 | Omeprazole (40mg/day).8 years | Loss of consciousness. Seizures | Mg++ 0.38Ca++ 6.87K+ 2.7 | ND/low | Electrolyte changes after lansoprazole and pantoprazole | Probable | Not specified |
| Kupiers et al. (2009) | M/76 | Esomeprazole (40mg/day).1 year | Lethargy. Cramps | Mg++ 0.43Ca++ 5.25K+ 3.3 | Normal/low | After esomeprazole, low Mg++ levels | Probable | Yes(6 weeks) |
| Gato Díez et al. (2011) | M/70 | Omeprazole (20–40mg/day).11 years | Supraventricular tachycardia. Tetany | Mg++ 0.48Ca++ 7.08 | Normal/normal | Giardiasis | Probable | Yes(4–6 weeks) |
| Hoorn et al. (2010) | M/63F/73F/62M/81 | Esomeprazole (20mg/day).11 yearsPantoprazole (40mg/day).1 yearOmeprazole (40mg/day).13 yearsEsomeprazole (20mg/day).3 years | Atrial fibrillationU wavesProlonged QTc. ST decreaseProlonged QT | Mg++ 0.06–1.88Ca++ 7.8K+ 2.8Mg++ 0.68–1.68Ca++ 11.5 *K+ 2.8Mg++ <0.16–1.8Ca++ 7.1K+ 2.7Mg++ 0.26–1.58Ca++ 4.44K+ 3 | Low/normalLow/normalNormal/normalNormal/normal | Loss of consciousnessSarcoidosisPrimary hyperparathyroidismPrior intestinal giardiasis. Lymphangiectasis. Rabeprazole | ProbableProbableProbableProbable | Yes(2 weeks)Yes(12 weeks)Yes(2 weeks)Yes(3 weeks) |
| MacKay and Bladona (2010) | 9F:1M/68.8 (±8.6) | Esomeprazole, omeprazole,pantoprazole8.3 (±3.5) years | Paresthesia (n=5). Cramps (n=3).Dizziness (n=5).Weakness (n=6).Tetany (n=2).Loss of consciousness (n=3) | Mg++ 0.63Ca++ 7.49 | Low 2/4 cases tested/Abnormally low in 2 | Concomitant diuretic use (n=8).Campylobacter enteritis (n=1) | Possible/probable | Yes(mean, 2 weeks) |
| Cano Megías et al. (2011) | M/40 | Omeprazole (40mg/day)5 years | Cramps. Tetany. Paresthesia | Mg++ 0.7Ca++ 7.6K+ 2.2P+ 1.8 | ND/normal | Concomitant thiazide use.Diarrhea. Lactose intolerance | Probable | Yes(3 weeks) |
F: female; ND: not done; M: male.
Mean values of a series of 10 patients. The Naranjo scale could only be applied to the single case reported, giving a score of probable. The Naranjo scale is one of the algorithms most commonly used to assess the causal relationship of a drug adverse reaction (DAR). (Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. The categories corresponding to the total score are as follows: DAR is: definite: > 9; probable: 5–8; possible: 1–4; unlikely <1.
Cases published in the medical literature report the concomitant occurrence of other electrolyte disorders such as hypokalemia (n=9, mean value 2.7mM/L) and hypophosphatemia (n=2).5,8 Secondary hypocalcemia was reported in all cases (mean Ca2+ values, 7.16mg/dL).
The mechanism of hypomagnesemia induced by PPIs has not yet been elucidated. Schlingman et al.9 proposed that treatment with PPIs induced changes in intestinal pH and caused a decreased expression of the family of transient receptor potential melastatin (TRPM) channels in heterozygous individuals. Two of these receptors, TRPM6 and TRPM7,9,10 which are essential for intestinal transepithelial and renal tubular transports of Mg++, have recently been identified. Their expression is regulated by several factors including Mg++ serum levels, angiotensin II, aldosterone, bradykinin, estrogens, drugs inducing hyperglycemia, diabetes mellitus, diuretics, acidosis or alkalosis, or immunosuppressants such as tacrolimus.
Complete recovery from water and electrolyte disorders usually occurred after a mean of 6 weeks (1–56 weeks). In almost two thirds of the patients (n=18), the PPI was replaced by an anti-H2, while drug discontinuation was the only action taken in the remainder. In conclusion, hypomagnesemia induced by long-term treatment with PPIs is a drug-class side effect. It may be triggered by multiple factors, which should be considered in each individual patient. MacKay and Bladon1 recommended annual electrolyte tests, including K+, Ca++, and Mg++, in all patients treated with PPIs. Hypomagnesemia is an electrolyte change fully reversible upon PPI discontinuation. However, most patients require several weeks of treatment with oral Mg++ and Ca++ supplements for complete resolution of electrolyte abnormalities. Clinicians should be aware of potential electrolyte changes in patients attending with tetany, arrhythmia, or seizures for an unknown cause and who are taking long-term treatment with PPIs.
Conflicts of interestThe authors state that they have no conflicts of interest.
To Dr. Pablo Guisado Vasco for his kindness and advice in preparation of this report.
Please cite this article as: Cano Megías M, et al. Hipomagnesemia relacionada con el uso de inhibidores de la bomba de protones, diarrea e intolerancia a lactosa. Endocrinol Nutr. 2011;58:550–5.