Eighty percent of patients with type 2 diabetes mellitus (T2DM) are overweight or obese, which in turn is associated with other cardiovascular risk factors and an increased risk of cancer. Large intervention studies that focused on intensive glycemic control have failed to show a reduction of cardiovascular events in T2DM patients. The two major concerns in these studies were weight gain and severe hypoglycemia in the arms of intensive intervention, which could have mitigated the potential beneficial effect of glycemic control. On the contrary, weight loss in diabetic patients through changes in lifestyle, drugs and/or surgery simultaneously improves all cardiovascular risk factors including hyperglycemia. Bariatric surgery has shown an early resolution of T2DM in a large percentage of patients and a decrease of diabetes-specific mortality. Despite this, all consensus and recommendations for the treatment of T2DM focus their decisions on the glycated hemoglobin value. This article aims to open a debate on the need to replace the glucose-centered therapeutic strategy for a weight-centered strategy.
El 80% de los pacientes con diabetes mellitus tipo 2 (DM2) tienen sobrepeso u obesidad, lo que se asocia a su vez con otros factores de riesgo cardiovascular y con un riesgo aumentado de cáncer. Los grandes estudios de intervención centrados en el control intensivo de la glucemia no han logrado demostrar una reducción de eventos cardiovasculares en pacientes diabéticos tipo 2. Los dos principales problemas observados en estos estudios son la ganancia de peso y la aparición de hipoglucemias graves en las ramas de intervención intensiva, lo que podría haber mitigado el potencial efecto favorable del control glucémico. Por el contrario, la pérdida de peso en pacientes diabéticos mediante cambios en el estilo de vida, fármacos y/o cirugía mejora simultáneamente todos los factores de riesgo cardiovascular, incluida la hiperglucemia. La cirugía bariátrica ha mostrado una rápida resolución de la DM2 en un gran porcentaje de pacientes y reduce la mortalidad específica para diabetes. A pesar de ello, todos los consensos y recomendaciones para el tratamiento de la DM2 centran la toma de decisiones en el nivel de hemoglobina glucosilada. Este artículo pretende abrir un debate sobre la necesidad de sustituir la estrategia terapéutica glucocéntrica por una estrategia adipocéntrica.
Type 2 diabetes mellitus (T2DM) is among the most significant comorbid conditions associated with central or visceral obesity, and the current worldwide increase in prevalence of T2DM runs parallel to the pandemic of obesity.1 The Nurses’ Health Study showed that relative risk of diabetes was 40-fold greater in women with a body mass index (BMI) higher than 35kg/m2 as compared to those with a BMI less than 23.2 From 1987 to 2007, the absolute prevalence of overweight in Spain increased by 14.1% in males and 10.3% in females, while obesity rates increased by 8.7% and 7.3% in males and females respectively. The end result was that in 2007, 61.6% of Spanish males and 46.0% of Spanish females had a BMI higher than 25; the corresponding obesity figures were 15.9 and 15.6% respectively.3 According to the most recent data published by the International Diabetes Federation, the prevalence of DM in the Spanish adult population is estimated at 8.7% in 2010, with a projected figure of 11.1% in 2030.1 However, a recent epidemiological study in Spain (Estudio di@bet.es) including 5419 subjects over 18 years of age from 100 healthcare centers showed an age- and sex-adjusted total prevalence of DM of 14.5%.4 This same study reported a 28% prevalence of obesity.5
Data from the US NHANES study showed that 80.3% of patients with T2DM have a BMI higher than 25kg/m2 and 49.1% BMI values higher than 30,6 which are in turn associated with other cardiovascular risk factors (CVRFs) such as high blood pressure (HBP), atherogenic dyslipidemia, microalbuminuria, and increased levels of proinflammatory and prothrombotic factors. Central obesity, defined as an increased waist/hip ratio, was shown to be strongly associated with myocardial infarction after adjustment for BMI in the INTERHEART case–control study.7 Anthropometric measures such as BMI or waist circumference (WC) probably underestimate the actual prevalence of central obesity, defined as increased abdominal fat, in the population with T2DM.
T2DM is also associated with an increased risk of certain cancers, such as liver, pancreas, endometrial, colonic, rectal, breast, and bladder tumors.8 This association may partly be due to risk factors shared by both diseases such as obesity, ageing, diet, and physical inactivity.
Weight loss through lifestyle changes simultaneously improves all CVRFs, including DM.9 Two large cohort studies conducted on morbid obese subjects undergoing bariatric surgery showed a reduction in overall mortality (particularly from cardiovascular disease and cancer) and in DM-specific mortality as compared to patients receiving conventional treatment for obesity.10,11 Comprehensive T2DM treatment should, therefore, be aimed at controlling all associated comorbidities and should include weight loss, and especially visceral fat loss, as an essential component. Large interventional studies focused on intensive glycemic control have failed to show a reduction in cardiovascular events in diabetic patients.12–14 The two main problems found in such studies were weight gain and the occurrence of severe hypoglycemia in the intensive intervention arms, which may have lessened the potential favorable effect of glycemic control. In addition, a recent meta-analysis of randomized clinical trials showed no cancer risk reduction with intensive glycemic control.15
A consensus document promoted by the Spanish Society of Diabetes, in collaboration with other scientific societies, on drug treatment of hyperglycemia in type 2 diabetes was published in September 2010.16 The main positive aspects of the document are individualization of control goals based on patient characteristics and stratification of treatment options by glycosylated hemoglobin levels (HbA1c). It has, however, the same problem as all consensuses and clinical guidelines published by various scientific societies: the decision algorithm is based on HbA1c.17–19 The treatment intensification approaches preferentially recommended by these documents often lead to an unwanted weight gain that has a negative impact on other comorbidities of obese patients.19
In short, the main question currently raised by physicians who care for type 2 diabetic patients is whether the old glucocentric treatment approach should be replaced by an adipocentric approach more consistent with the pathophysiology of the disease, along with strict control of other cardiovascular risk factors. The purpose of this article is to stimulate a debate which will lead to the establishment in the not too distant future of a new philosophy for the management of patients with increased abdominal fat for who also have diabetes.
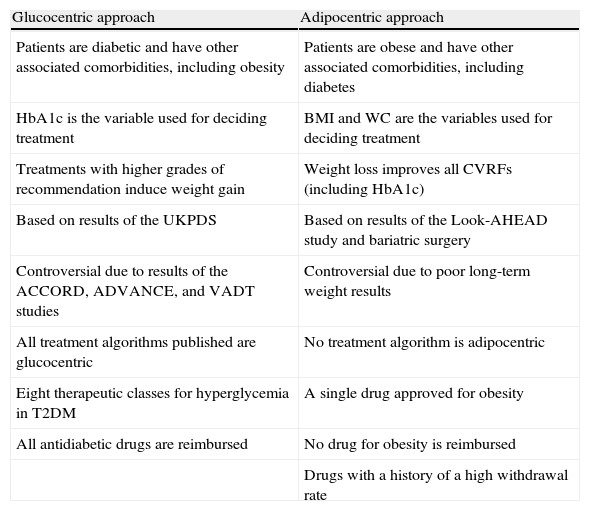
Glucocentricity or adipocentricity?Table 1 shows the main differences between the two types of treatment. Advocates of the glucocentric approach base their position on the results of the well-known interventional United Kingdom Prospective Diabetes Study (UKPDS).20,21 This study, conducted on patients with newly diagnosed T2DM, showed that improved glycemic control with drug treatment was associated with a decreased risk of microvascular complications (retinopathy, neuropathy, and nephropathy). A 16% reduction in cardiovascular complications was also seen in the intensive control arm, but the difference was not statistically significant. Ten-year follow-up of the UKPDS cohort after trial completion showed that patients initially randomized to intensive control had long-term reductions in myocardial infarction (15% with sulfonylureas or insulin as initial treatment, and 33% with metformin as initial treatment, both statistically significant) and all-cause mortality (13% and 27% respectively).22 This has been attributed to the effect of so-called hyperglycemic memory during the first years of treatment.
Characteristics of the glucocentric and adipocentric approaches to the management of patients with T2DM.
| Glucocentric approach | Adipocentric approach |
| Patients are diabetic and have other associated comorbidities, including obesity | Patients are obese and have other associated comorbidities, including diabetes |
| HbA1c is the variable used for deciding treatment | BMI and WC are the variables used for deciding treatment |
| Treatments with higher grades of recommendation induce weight gain | Weight loss improves all CVRFs (including HbA1c) |
| Based on results of the UKPDS | Based on results of the Look-AHEAD study and bariatric surgery |
| Controversial due to results of the ACCORD, ADVANCE, and VADT studies | Controversial due to poor long-term weight results |
| All treatment algorithms published are glucocentric | No treatment algorithm is adipocentric |
| Eight therapeutic classes for hyperglycemia in T2DM | A single drug approved for obesity |
| All antidiabetic drugs are reimbursed | No drug for obesity is reimbursed |
| Drugs with a history of a high withdrawal rate |
WC, waist circumference; CVRFs, cardiovascular risk factors; HbA1c, glycosylated hemoglobin; BMI, body mass index.
The UKPDS cannot be considered as an intensive interventional study in the current sense of the term, because the group randomized to conventional therapy initially received dietary measures only, and the intensive treatment arm would now be called conventional treatment, but it showed that good glycemic control from the time of diagnosis of T2DM provided long-term cardiovascular benefits. However, the results of three large trials (ACCORD, ADVANCE, and VADT)12–14 showed no significant improvement in cardiovascular prognosis with intensive glycemic control in patients with more advanced T2DM than participants in the UKPDS. In fact, the ACCORD study was terminated early due to a 22% increase in overall mortality in the intensive control group.12 Subgroup analysis in these trials suggested a benefit of intensive glycemic control on cardiovascular disease in patients with shorter T2DM duration, lower initial HbA1c levels and/or the absence of known cardiovascular disease.
Although weight gain induced by intensive glycemic control has not been shown to reduce the potential benefit of glycemic control on cardiovascular morbidity and mortality, weight increase may influence results through difficult to quantify intermediate variables due to the known interrelations of obesity with CVRFs. Mean weight increases of 4kg in the insulin arm of UKPDS,20 3.5kg in the intensive arm of ACCORD,12 and 7.8kg in the intensive arm of VADT14 were found. The only study showing no weight increase with intensive glycemic control was ADVANCE,13 probably because of the lower insulinization rate.
For “glucocentric” physicians, patients are diabetic with other associated comorbidities, including obesity. They have 8 different drug classes available for treating hyperglycemia, all reimbursed by the National Health System, and follow treatment algorithms often leading to significant weight increases in patients within a few years because of the preferential use of sulfonylureas, glinides, glitazones, or insulin. Patients are exposed to an occurrence of hypoglycemia, which may be serious. Fortunately, guidelines recommend metformin as initial drug treatment. The benefits of metformin on glycemic control and the reduction of microvascular and macrovascular complications21 are associated with a neutral effect on weight, and the drug may also have other beneficial therapeutic properties, such as tumor risk reduction, which are being investigated,8 The use of dipeptidyl peptidase-4 inhibitors (DPP4Is), which also has a neutral effect on weight, in the second treatment step is also increasing. Another positive aspect of “glucocentric” guidelines is the recommendation of strict control, often with drugs, of other CVRFs such as HBP, dyslipidemia, and smoking.23
By contrast, for adipocentric physicians, patients with T2DM are overweight/obese patients who also have other associated comorbidities, including diabetes. Weight loss simultaneously controls all CVRFs and decreases a priori the need for drug treatment. This therapeutic approach runs into many difficulties. The main parameters considered for deciding treatment are BMI and WC, but no algorithm reported uses these as primary criteria, although it is true that guidelines include obesity as a secondary criterion for selecting treatment after metformin. Treatment options include lifestyle changes (LSCs), drug treatment, and bariatric surgery; the latter option is not included in any treatment algorithm. Only one of the eight drug classes for the treatment of hyperglycemia in T2DM, glucagon-like peptide-1 (GLP-1) receptor agonists induces a significant weight loss, and the only drug currently approved in Spain for obesity is orlistat, which like all drugs of this type is not reimbursed and is seldom included in therapeutic guidelines for T2DM. Finally, drugs for obesity are considered “suspicious” by regulatory agencies, health authorities, and physicians themselves, who still consider obesity as a cosmetic problem not justifying the potential risk of a new drug. The problem is aggravated by the high number of weight-reducing drugs withdrawn from the market in recent years (dexfenfluramine, rimonabant, and sibutramine) and the indiscriminate use of such drugs by the general population for purely cosmetic purposes.
Lifestyle changes in T2DMLSCs are an essential component of the treatment of hyperglycemia and all other CVRFs in patients with T2DM. However, long-term maintenance of LSCs in diabetic patients is very difficult in clinical practice. Weight loss with LSCs and/or drug treatment for obesity is particularly difficult in obese patients with T2DM.24 In addition, as previously stated, most drugs used in T2DM to treat hyperglycemia induce weight increase or have at the most a neutral effect. A systematic review of 22 randomized clinical trials in T2DM assessing the effects of LSCs or behavioral therapy as compared to standard treatment after follow-up periods of 1–5 years showed a weight difference of only 1.7kg (95% confidence interval [CI], 0.3–3.2kg).25
The Look AHEAD (Action for Health in Diabetes) study was a large clinical trial designed to assess whether long-term weight loss with LSCs improved glycemic control and prevented cardiovascular disease in patients with T2DM.9 One-year follow-up data from this study (with a planned total duration of 11.5 years) confirmed that, in T2DM patients, an intensive lifestyle intervention including calorie restriction, mainly at the expense of fat, and moderate to intense physical activity achieved a mean weight loss at one year of follow-up of 8.6% in the intervention arm, as compared to 0.7% in the control arm. More importantly, intervention also achieved decreases in HbA1c (from 7.3% to 6.6%), blood pressure, HDL (high density lipoprotein) cholesterol, triglycerides and microalbuminuria, and a reduction in the number and/or dose of drugs for diabetes, hypertension, or dyslipidemia. The recently reported results of a four-year study showed significant, although smaller differences in weight, HbA1c, and other risk factors, except for LDL (low density lipoprotein) cholesterol, due to a greater use of statins in the control arm.26 The critical question is whether the differences seen between both groups will result in a reduction in cardiovascular disease, but we must wait some years for these results to be available However, the effects on risk factors such as those reported in the Look AHEAD study have been associated in prior clinical trials and observational studies with a decrease in cardiovascular morbidity and mortality.
The protocol of the Look AHEAD study is very difficult to use in actual clinical practice.9 Study participants were seen weekly for the first six months, with three group meetings and one individual visit per month; in the following six months they attended bimonthly group meetings and paid monthly individual visits. Patients subsequently returned for individual visits at least once monthly. The management team comprised dieticians, psychologists, and specialists in physical exercise.
In Spain, however, we have a potent treatment tool based on age-old tradition, the Mediterranean diet, which has been shown to be as effective for weight loss in obese patients as low carbohydrate or low fat diets, and to decrease the incidence of diabetes by 52% as compared to a low fat diet.27,28 In patients with newly diagnosed T2DM, the Mediterranean diet improves glycemic control and other CVRFs as compared to a low fat diet and also reduces the need for antidiabetic drugs.29 An Australian cohort study showed reductions in total and DM-related mortality in patients with greater adherence to a Mediterranean diet pattern.30
A current advocating a multifactorial approach to diabetic patients has emerged in recent years. This approach is based on the results of the Steno study.31 In patients with T2DM and microalbuminuria, treatment intensification including LSCs, smoking cessation, hypoglycemic treatment to decrease HbA1c under 6.5%, lipid-lowering treatment to maintain total cholesterol levels less than 175mg/dL, antihypertensive treatment (with routine use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) to achieve blood pressure levels less than 130/80mmHg, aspirin, and vitamin supplements decreased the risk of cardiovascular disease and microvascular complications by 50% as compared to the conventional treatment arm, although few patients achieved HbA1c levels <6.5%, and there was even a slight weight gain in both groups. Observational follow-up after trial completion showed reductions in all-cause and cardiovascular mortality in the intensive treatment arm.32 Using cardiovascular risk calculators, the authors concluded that the use of statins and antihypertensives had the greatest risk reduction effect. This study confirms observations in daily clinical experience suggesting that weight control and glycemic goals are much more difficult to achieve in T2DM than blood pressure and lipid goals. A pragmatic view of the study would suggest that we should concentrate on blood pressure and lipid control, and to a lesser extent on glycemic control, giving up intensive weight intervention. In my opinion, however, the message to be taken is: antihypertensive and lipid-lowering drugs are synergistic with weight loss for decreasing cardiovascular risk, but we could probably avoid the use of multiple drugs if patients achieved weight goals.
Optimal drug treatment in patients with T2DMBecause of the results of the UKPDS, metformin is the first choice antidiabetic treatment in all guidelines, even with concomitant LSCs, because of the decreases seen in all-cause and DM-specific mortality, microvascular complications, and myocardial infarction.21 Metformin causes no hypoglycemia and was the drug inducing the least weight gain in the UKPDS. Some guidelines even recommend the use of metformin for the prevention of T2DM in patients at a high risk of progression to DM, such as those with altered basal blood glucose and/or glucose intolerance, HbA1c higher than 6%, and no response to lifestyle changes.23 A total lack of agreement exists, however, about the treatment step after metformin in T2DM. Many algorithms recommend sulfonylureas essentially because they are cheaper, a lot of experience has been acquired regarding their use, and they have achieved a reduction in microvascular complications as a monotherapy.20 However, it should not be forgotten that in the UKPDS an unexpected increase occurred in mortality related to DM with the combination of metformin and sulfonylurea,21 a finding that has not been adequately clarified yet. Early insulin use is also advocated because it is the most effective hypoglycemic drug and for its protective effect on microvascular complications,20 but insulin, like sulfonylureas, induces weight gain and increases the risk of hypoglycemia. The risk of hypoglycemia is lower with new basal insulin analogues, glargine and detemir, as compared to insulin NPH, and detemir appears to induce less weight gain than NPH.33 A recent consensus document jointly published by the American Diabetes Association and the American Cancer Society8 concluded that early, limited data suggested that metformin could be associated with a lower risk of cancer and that exogenous insulin could be positively associated with a risk of neoplasms. However, it warned that studies were needed to clarify whether such an association was causal and, in addition that controversial epidemiological data suggesting a higher risk with glargine would need to be confirmed in well designed studies.
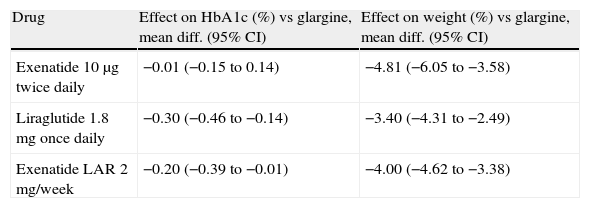
The alternative adipocentric proposal recommends the use of GLP-1 receptor agonists such as exenatide, liraglutide or, in the near future, once-weekly preparations such as exenatide LAR, albiglutide, taspoglutide, or semaglutide.34 These drugs induce weight loss mediated by a central anorexigenic effect and delayed gastric emptying, long-term glycemic control improvement induced by the incretin effect and weight loss, and reduction in other CVRFs such as blood pressure or lipids. Their efficacy is similar or greater than that of basal insulins or other oral antidiabetics (Table 2), with the added advantage that they do not induce hypoglycemia.35 There has been speculation about the potential protective effect on the beta cells of the inhibition of apoptosis, which would change the natural history of the disease, but we will have to wait several years before this can be verified. It is currently unknown whether the beneficial effects on multiple factors will result in a long-term reduction of cardiovascular risk. The main limitations of this therapeutic class are the administration route, the cost, the gastrointestinal side effects, the difficulty in predicting the profile of patients who will achieve a good glycemic and weight response, and a lack of experience concerning long-term safety.
Effect on glycosylated hemoglobin (HbA1c) and body weight of glucagon-like type 1 (GLP-1) receptor agonists, as compared to basal insulin therapy, in patients with T2DM.
| Drug | Effect on HbA1c (%) vs glargine, mean diff. (95% CI) | Effect on weight (%) vs glargine, mean diff. (95% CI) |
| Exenatide 10μg twice daily | −0.01 (−0.15 to 0.14) | −4.81 (−6.05 to −3.58) |
| Liraglutide 1.8mg once daily | −0.30 (−0.46 to −0.14) | −3.40 (−4.31 to −2.49) |
| Exenatide LAR 2mg/week | −0.20 (−0.39 to −0.01) | −4.00 (−4.62 to −3.38) |
95% CI, 95% confidence interval.
A drug that could be used more frequently to treat diabetic patients with overweight is orlistat, a pancreatic lipase inhibitor often forgotten due to the lack of reimbursement and the sensation that it has no significant effect on glycemic control or weight in T2DM. However, a meta-analysis of controlled clinical trials in diabetic patients with follow-up periods longer than one year showed a mean weighted difference from placebo of −2.61% of body weight (95% CI, 3.06–2.17).24 A review of pooled data from 7 clinical trials showed that orlistat caused a significant HbA1c decrease of 0.74%, as compared to 0.31% with placebo.36 This glycemic control improvement is greater than expected for the weight loss seen. Several alternative mechanisms have been postulated to explain this effect, such as improved insulin sensitivity, slower and incomplete digestion of dietary fat, decreased postprandial blood lipid levels, reduced visceral adipose tissue, and the stimulation of intestinal GLP-1 secretion.
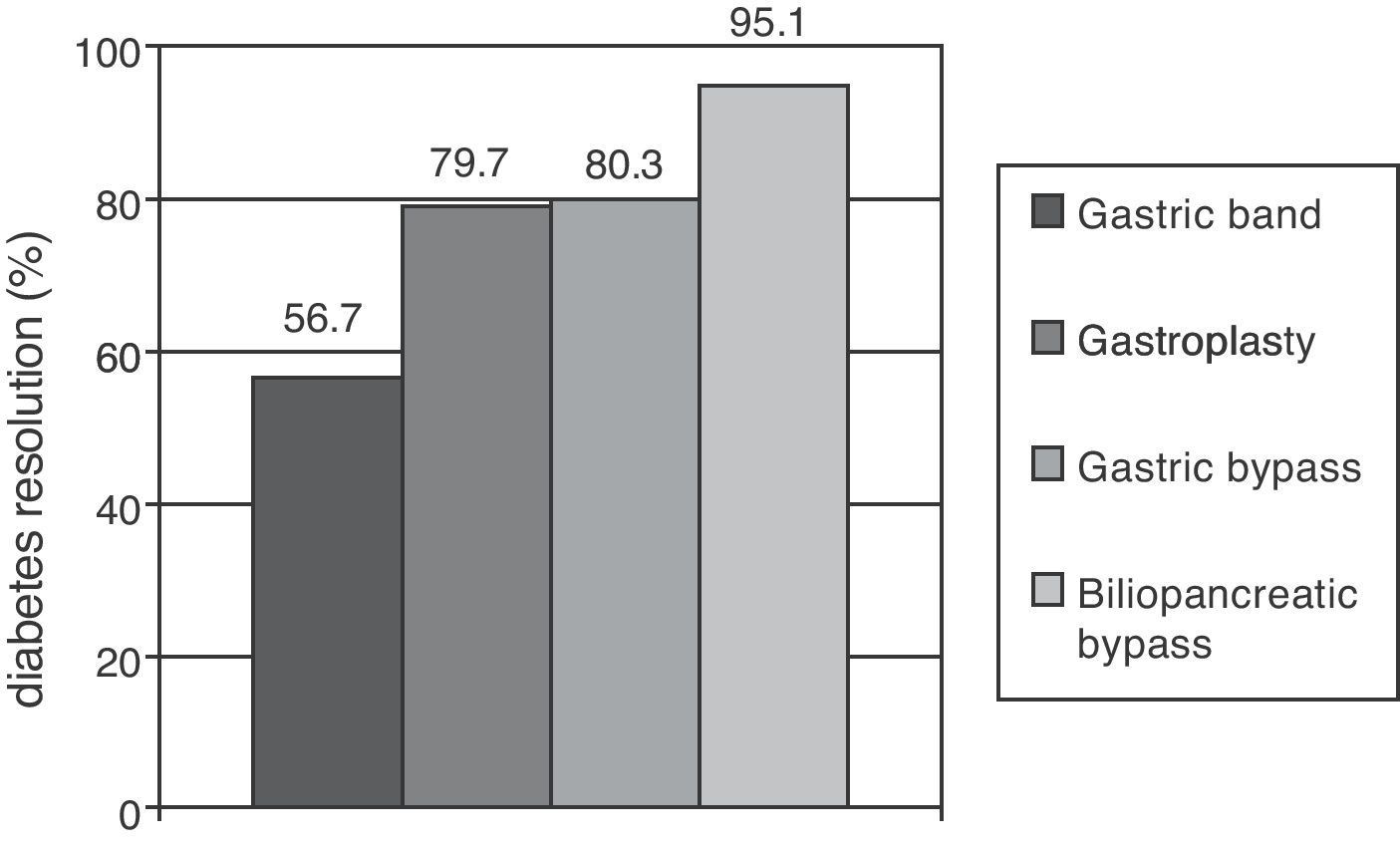
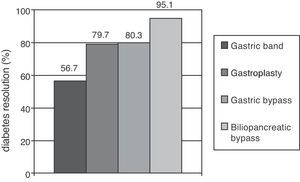
Metabolic surgery in patients with T2DMFocusing on weight loss, the most dramatic results in the treatment of T2DM have been achieved in patients undergoing bariatric surgery, especially with malabsorptive procedures. Clinical practice guidelines recommend this procedure to patients with BMI higher than 40 who do not lose weight with dietary and pharmacological measures, and extend the indication to patients with a BMI higher than 35 and major comorbidities such as T2DM, HBP, dyslipidemia, cardiovascular disease, severe osteoarthritis, or sleep apnea.37 A recent meta-analysis showed that gastric and biliopancreatic bypasses achieved long-term resolution of diabetes in 80.3% and 85.1% of patients respectively (Fig. 1).38 The main objection to these results is the poor methodological quality of the studies considered. Glycemic control improvement is achieved a few days after surgery, when a significant weight loss has not yet occurred. It is therefore thought that anatomical bowel modification induces a change in the secretion of gastrointestinal peptides (incretins and anti-incretins) which is partly responsible for the resolution of T2DM in many of these patients.39
Proportions of patients undergoing different bariatric surgery procedures who achieved resolution of diabetes mellitus after follow-up periods longer than two years. Resolution of type 2 diabetes (T2DM) was defined as withdrawal of all drugs for T2DM with basal blood glucose levels less than 100mg/dL and/or glycosylated hemoglobin levels less than 6%.
The relative weight of weight loss and incretin effect on the resolution or improvement of T2DM after bariatric surgery is currently unknown. A clinical trial compared the laparoscopic adjustable gastric band (a restrictive procedure inducing no significant changes in incretins) to conventional medical treatment in patients with T2DM and BMI ranging from 30 and 40.40 Surgical patients lost 20.7% of their initial weight, and 73% of them showed remission of T2DM at two years of surgery; control patients lost 1.7% of their initial weight and 13% met remission criteria at the end of the study. Calorie restriction and weight loss are therefore likely to play a central role in the improvement of carbohydrate metabolism in patients undergoing bariatric surgery.
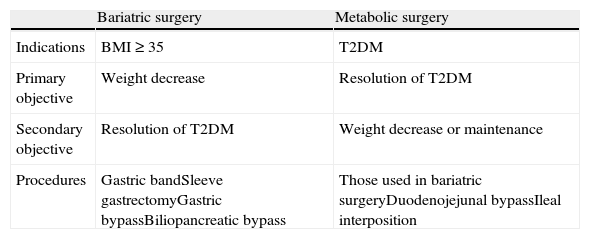
Bariatric surgery not only achieves resolution or a clear improvement of T2DM, but also improves multiple CVRFs, and also decreases all-cause mortality, as noted above.10,11 It is therefore not surprising that attempts have been made to extend the indication of gastrointestinal surgery not only to obesity-associated diabetes but also to T2DM itself, which has led to the concept of metabolic surgery (Table 3).41 This practice, which has not been validated by clinical trials or included in treatment guidelines, involves the use of both conventional bariatric procedures and experimental gastrointestinal procedures aimed at increasing incretin secretion (ileal) or preventing the secretion of hypothetic anti-incretin factors (duodenojejunal bypass).42
Differences between bariatric and metabolic surgery.
| Bariatric surgery | Metabolic surgery | |
| Indications | BMI≥35 | T2DM |
| Primary objective | Weight decrease | Resolution of T2DM |
| Secondary objective | Resolution of T2DM | Weight decrease or maintenance |
| Procedures | Gastric bandSleeve gastrectomyGastric bypassBiliopancreatic bypass | Those used in bariatric surgeryDuodenojejunal bypassIleal interposition |
T2DM, type 2 diabetes mellitus; BMI, body mass index.
Metabolic surgery for T2DM has many detractors, who consider it a draconian way of treating a medical disease. An integrated review of previous studies which had reported on metabolic surgery in diabetic patients with a BMI less than 35 and very heterogeneous follow-up (mean, 23 months; range, 6–216 months) concluded that 89.1% of patients with prior BMI ranging from 30 to 35 and 81.8% of patients with prior BMI ranging from 25% to 29.9 discontinued all drugs for DM after surgery and maintained close to normal mean blood glucose values. Mortality was very low (0.29%), and BMI decrease was moderate, −6.8 and −3.4 in the obese and overweight groups, with mean final BMI values of 25.2 and 23.1 respectively.43 If these data are confirmed in currently ongoing clinical trials, BMI should not be a decisive factor when selecting candidates for metabolic surgery.42 Several studies have shown that metabolic surgery may be a cost-effective procedure for the treatment of T2DM.44
An international conference on gastrointestinal surgery in T2DM with the participation of multiple scientific societies concluded that gastrointestinal surgery using gastric bypass, adjustable gastric band, or biliopancreatic bypass could be considered as a treatment for T2DM in acceptable surgical candidates with a BMI greater than 35 and poorly controlled as to LSCs and medical treatment.45 Surgery may also be appropriate as an alternative in poorly controlled diabetic patients with BMIs ranging from 30 to 35. Gastric bypass is the procedure of choice in this latter group. While new surgical procedures such as duodenal–jejunal bypass, ileal interposition, sleeve gastrectomy, or intraluminal cuff have shown encouraging results in preliminary studies, they should only be used in clinical trials.
In any case, long-term, well-designed studies which demonstrate the efficacy and safety of metabolic surgery in patients with a BMI less than 35 are needed before it can be considered as an alternative to medical treatment. These procedures should therefore not be performed outside clinical trials or, exceptionally, in highly selected cases as compassionate treatment.
Conclusion: Is there a need for a change in the treatment model in T2DM?Advocates of the dominant glycemic model would argue that no change is needed in the treatment model because current algorithms are able to improve glycemic control and reduce microvascular complications. In a patient subgroup, intensive glycemic control may also decrease macrovascular complications. In addition, the weight increase experienced by patients over time has not been shown to be a negative prognostic factor.
In contrast to this orthodox position, supporters of the adipocentric model like us think that the glucocentric approach does not achieve long-term control of blood glucose in a relevant proportion of patients even with complex insulin therapies. Thus, in the 4T study, 32.6–50.6% of patients on intensive insulin therapy did not achieve HbA1c levels lower than 7% at three years.46 Drugs recommended as second-line treatment such as sulfonylureas, glinides, or insulin induce hypoglycemia, and both these drug classes and glitazones promote weight increase. In many cases, weight gain worsens other CVRFs and various comorbid conditions in obese patients, such as sleep apnea, hypoventilation, osteoarthritis, or heart failure. There is increasingly worrying evidence about the association of obesity, diabetes, and cancer, and weight increase may close this vicious circle. Finally, the classical model eventually depletes the pancreatic reserve, and patients require insulin therapy sooner or later.
By contrast, the adipocentric approach acts upon the pathophysiological nucleus of the disease, decreasing the need of drugs for hyperglycemia and other CVRFs by LSCs. The great weight losses achieved with bariatric surgery decrease all-cause mortality and induce an early change in the natural history of the disease. Drugs with incretin effects may also be able to change the course of T2DM.
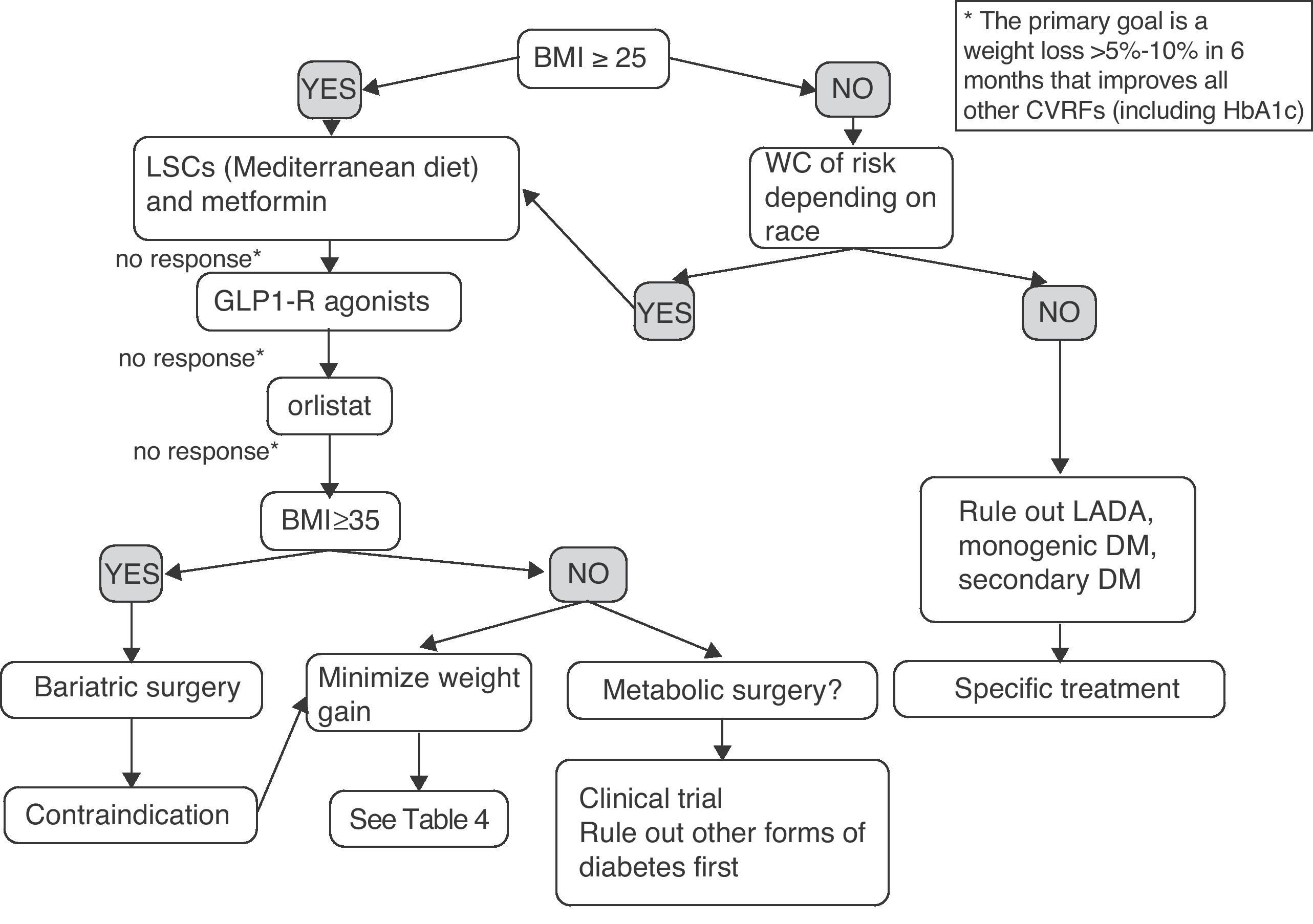
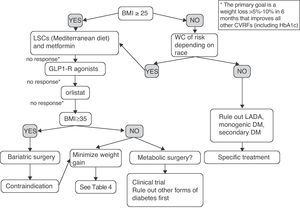
Fig. 2 shows a preliminary and empirical proposal for an adipocentric approach to the treatment of T2DM. It should be noted that the algorithm provided is not supported by clinical trials (it is based on the studies discussed in the article and on the author's opinion), and is therefore a scheme for an in-depth discussion, which is a pre-requisite before it may be proposed as a recommendation. Patients with prior diagnosis of T2DM but BMI and WC in the normal range probably have other forms of DM such as latent autoimmune diabetes of the adult (LADA), or monogenic or secondary DM, requiring specific treatment and for which the treatment model proposed would not be applicable. The decision would mainly be determined by weight loss and, secondarily, by improvement in all other CVRFs. The progressive combination of LSCs, metformin, GLP-1 receptor agonist, and orlistat is synergistic in its weight and glycemic goals, and should be offered to more patients. Moreover, all patients with T2DM and BMI greater than 35 have the right to know the effectiveness, cost-effectiveness ratio, and beneficial effects on morbidity and mortality of the surgical option. Finally, an indication of metabolic surgery may no longer be conditioned by BMI in the next few years. Some patients with BMI ranging from 30 and 35 and poor metabolic control despite multiple therapies could now be enrolled in controlled clinical trials at reference centers.
Empirical proposal of an adipocentric algorithm for the treatment of patients with T2DM. WC, waist circumference; CVRFs, cardiovascular risk factors; GLP1-R, glucagon-like type 1 peptide receptor; HbA1c, glycosylated hemoglobin; BMI, body mass index; LADA, latent autoimmune diabetes of the adult; LSCs, lifestyle changes.
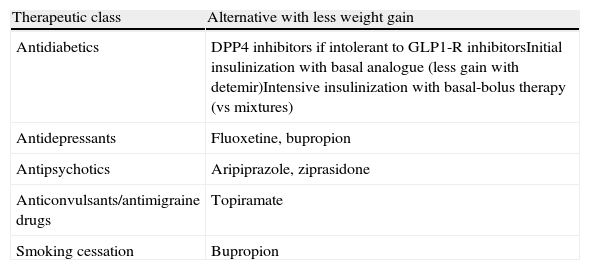
CVRFs will not be controlled with this algorithm in a variable proportion of patients. If this occurs, antidiabetic drugs with the least unfavorable effect upon patient weight will be used, and lipid-lowering and antihypertensive drugs will be added. It is also important to provide guidance to other specialists about drugs that may help their patients lose weight (Table 4).
Recommendations for minimizing weight gain in patients with T2DM not achieving glycemic control goals or requiring other drug treatments.
| Therapeutic class | Alternative with less weight gain |
| Antidiabetics | DPP4 inhibitors if intolerant to GLP1-R inhibitorsInitial insulinization with basal analogue (less gain with detemir)Intensive insulinization with basal-bolus therapy (vs mixtures) |
| Antidepressants | Fluoxetine, bupropion |
| Antipsychotics | Aripiprazole, ziprasidone |
| Anticonvulsants/antimigraine drugs | Topiramate |
| Smoking cessation | Bupropion |
DPP4, dipeptidyl peptidase 4; GLP1-R, glucagon-like type 1 peptide receptor.
The patients seen may have type 2 diabetes with other associated comorbid conditions, including obesity, or may be obese and have other comorbidities such as diabetes. It may appear a somewhat banal comment, but if we become accustomed to using the second formula in our diagnoses, we will have initiated a profound change in the management of patients with T2DM.
Conflicts of interestJ.J. Gorgojo Martinez states that he has received honoraria for lectures, training activities, and research work from the following pharmaceutical companies: Novo Nordisk, Lilly, Sanofi-Aventis, Roche, GlaxoSmithKline, Pfizer, Almirall, Novartis, Abbott, MSD, and Bristol-Myers Squibb.
Please, cite this article as: Gorgojo Martínez JJ. Glucocentrismo o adipocentrismo: una visión crítica de los consensos y guías clínicas para el tratamiento de la diabetes mellitus tipo 2. Endocrinol Nutr. 2011;58:541–9.