Incidence and prevalence of overweight and obesity have greatly increased over the past three decades in almost all countries around the world. This phenomenon is not easily explained by lifestyle changes in populations with very different initial habits. This has led to consider the influence of other factors, the so-called endocrine disruptors, and more specifically obesogens. This study reviewed the available evidence about polluting chemical substances which may potentially be obesogens in humans: DES, genistein, bisphenol A, organotins (TBT, TPT), and phthalates. The first three groups of substances mainly act upon estrogen receptors, while organotins and phthalates activate PPARγ. It was concluded that evidence exists of the obesogenic effect of these chemical substances in tissues and experimental animals, but few data are available in humans.
La incidencia y prevalencia de sobrepeso y obesidad ha experimentado un gran incremento en las últimas tres décadas y afecta a casi todos los países del orbe. Este fenómeno no se explica fácilmente por los cambios del estilo de vida en las distintas poblaciones con hábitos de partida muy distintos. Por lo que además del cambio del estilo de vida, otros factores empiezan a tenerse en cuenta, los llamados disruptores endocrinos y más concretamente los obesógenos. Revisamos la evidencia que existe sobre sustancias químicas que polucionan el ambiente que potencialmente puedan ser obesógenos en humanos: el dietilestilbestrol (DES), la ginesteína, el bisfenol-A, los derivados orgánicos de estaño y los ftalatos. Los tres primeros actúan principalmente sobre los receptores estrogénicos y los derivados orgánicos del estaño y los ftalatos activando los PPARγ. En conclusión, existen evidencias del efecto obesógeno de estas sustancias en estudios en animales de experimentación, tanto in vitro como in vivo, pero muy pocas en humanos.
Incidence and prevalence of obesity, defined as an increase in adipose tissue, have increased in virtually all populations in the world, including the poorer countries,1 and Spain is not an exception.2–6 This has occurred in a relatively short time. Obesity is considered to occur as the result of an imbalance between calorie intake and energy expenditure in the body. Multiple biological and behavioral factors, as well as genetic factors, are involved in energy balance. The traditional explanation is that, since the middle of the past century, the population, mainly in developed countries, has increased its calorie intake. This, together with progressively sedentary lifestyles related to technological advances, particularly in children, has led to an imbalance resulting in an increased energy deposit as fat.7,8 There are however data that do not support the concept that the above factors are the cause of the increased prevalence and incidence of people with overweight and obesity in different populations with different lifestyles. A decrease in calorie intake by the population was shown in some industrialized countries during the past century.9 As regards physical activity, despite the increase during the past century in recreational activities with less energy expenditure such as TV and video games, the improvement in motorized transport, and use of tools that facilitate work at home, there is no evidence that this decreased physical activity is sufficient to explain increased obesity in the different populations.10,11 Finally, weight control is not simply a balance between intake and expenditure, but is rather the result of a complex phenomenon11 where body weight would homeostatically be regulated by certain genetically predefined mechanisms that would allow for maintaining stable a given body weight for a long time period.12
The explosive increase in prevalence of obesity in the populations in recent decades would be explained by the lack of ability of the body to adequately compensate for the modern lifestyle, characterized by excess calorie intake and limited physical activity. The thrifty phenotype hypothesis13 postulates that in the evolutionary historical stage we live, genetic traits would not adequately adapt to the current obesogenic environment.
Since none of the abovementioned potential causes fully explain the so-called obesity epidemic, other factors that may play a complementary role have been suggested, including eating behavior disorders, whose incidence and prevalence have increased in the population, particularly the less well known forms such as the night eating syndrome, pica syndrome,14 the high prevalence of attention deficit hyperactivity disorder in children and adults,15 and sleep disturbances.16
From the beginning of the past decade it has been suggested that toxic chemical agents may contribute to the increased frequency of obesity in the population. Baillie-Hamilton,17 after analyzing the correlation between increased frequency of obesity in the population and increased production of industrial chemical substances, proposed in 2002 the hypothesis of a causal relationship between both events. The term obesogens was subsequently coined to designate chemical substances that pollute the environment and that, incorporated into the body, may interfere with energy regulation and storage.
This review is intended to gather the available evidence on obesogens and their potential role in obesity development in humans.
The concept of endocrine disruptionIn 1996, Colbron et al.18,19 defined the term endocrine disruption for toxic agents that pollute the environment and substances contained in diet that inadequately modulate the neuroendocrine system. Some questions then arose such as what agents could cause detectable effects with routine exposure in animals and/or humans, and how risk of exposure to a catastrophic, negligible, or not proven disruptor could be quantified. It was subsequently recommended to reserve the term endocrine disruptor for chemical agents that affect such system through interaction with a receptor, on synthesis or elimination of the hormone.20 Multiple studies conducted in vitro, in experimental animals, and in humans in the past decade showed that many xenobiotics present in the environment and/or diet may interfere with the complex mechanism of neuroendocrine signaling, causing adverse effects.21–24 Exposure to chemical agents with estrogenic effects that would alter reproductive function initially caused concern. Xenobiotic agents were subsequently shown to have other mechanisms of action, and interest arose in chemical agents that mimic or interfere with the normal action of virtually all hormones, including estrogens, androgens, progestogens, thyroid hormones, and hypothalamic and pituitary hormones.25,26
Mechanism of action of endocrines disruptorsEndocrine disruptors (EDs) act by binding to any type of receptor, either nuclear, membrane, neurotransmitter (serotonin, dopamine, and norepinephrine receptors), and even orphan (aryl hydrocarbon) receptors. They also act on metabolic pathways of steroids in the reproductive system,24 as occurs with dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs), polybrominated biphenyl) (PBB), and some plant estrogens, which act upon estrogen receptors to alter sexual and reproductive behavior. Some diethylstilbestrols (DES) act as hormone antagonists; for instance, vinclozolin and dichlorodiphenyldichloroethylene (DDE),27 a DDT metabolite, contain substitutions of halogen, chlorine, and bromine groups with phenol radicals mimicking native steroid hormones and interact with receptors as agonists or antagonists. DES may also act by modifying synthesis and catabolism of natural hormones, increasing or decreasing their levels. Examples include phytoestrogens (flavones, isoflavones, coumestans, lignanes) and mycoestrogens (zeranol), which promote development of mammary glands in males.28
A challenge in the field of endocrine disruptors is the variety of substances that may even not have structural similarity to physiological regulators, except for their low molecular weight. Factors significant in endocrine disruption include: (a) Age at exposure, with a greater vulnerability to its effects during intrauterine development.27 (b) Latency time since exposure; the consequences of exposure in early developmental stages may become evident in adulthood or even in old age. (c) The significance of multiple contamination; contamination by a single environmental pollutant is rare, and additive or synergistic effects may occur.28 (d) Dose–response does not follow the traditional dynamics as, surprisingly, a pollutant may have greater effects at low as compared to high doses.29,30 (e) The transgenerational and epigenetic effect of endocrine disruptors, which may not only affect an individual, but also his/her offspring and even subsequent generations.31 (f) The diversity and complexity of mechanisms of action; endocrine disruptors often act by more than one mechanism, and metabolic products of disruptor breakdown also have effects different from those of the initial chemical substance.32
As regards the molecular effects of endocrine disruptors, these are thought to mimic or block transcription induced by natural steroids on their nuclear receptors. Among other effects, they have been shown to inhibit action of histone deacylase, alter DNA methylation, activate phosphorylation of coactivators such as p160, and reduce degradation of the disruptor–receptor complex, increasing its effect.33
ObesogensObesogens are xenobiotics that may occur in the environment and/or food and that inappropriately regulate and promote lipid accumulation and adipogenesis.34 Several recent studies have addressed the potential impact of the endocrine disruptor effect of the energy regulation system, i.e. obesogens and their relation to the increase in obesity prevalence in virtually all countries of the world.35–37
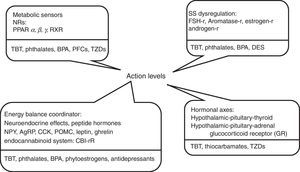
There are some indispensable conditions for the model of obesogen interaction with population health to be plausible. First, molecular targets of obesogens should be detected in adipose tissue. Second, obesogens should be able to alter the biology of adipose tissue, inducing increases in fat content of cells (hypertrophy) and cell number (hyperplasia). Third, a realistic relation should exist between contamination and effect, i.e. disruptor levels in the body should be able to induce a specific effect on energy balance. Finally, epidemiological studies should demonstrate the causal relationship of obesogens in a significant part of the population.
An additional significant aspect is the time when the chemical agent acts on our body and has an impact on induction of adipogenesis. There is current evidence that the intrauterine developmental period is most vulnerable to obesogens for the specific effect of promotion of adipogenesis in adults.36,38
DES exposure in the fetal or neonatal stages of development is clinically more relevant as compared to adult exposure, because organisms in such stages are extremely vulnerable to chemical substances with hormone-like effects. DES has been shown to have stronger effects at lower levels in the early stages of development.39 The reason for this is that the protective effects operating in adults, such as DNA repair, a competent immune system, detoxifying liver enzymes, and blood–brain barrier are not fully functional in the fetal and neonatal stages. Moreover, metabolism is increased in the fetal and antenatal stages as compared to subsequent developmental periods, which may enhance the effect of DES.
The concept that adult health and disease have their etiological basis in the fetal or neonatal stage is not unique to the field of endocrinology. At the end of the 80s, maternal nutrition studies suggested that fetal environment, as reflected in low birth weight and poor nutrition, was related to an increased risk of suffering non-transmissible diseases, cardiovascular disease, diabetes mellitus, osteoporosis, etc., which suggested the hypothesis diseases had their origin in fetal and neonatal stages.38
Fig. 1 shows some substances currently investigated for their obesogenic properties, and evidence available in current literature about some obesogenic potentials are reviewed below.
The diethylstilbestrol modelDES is a synthetic estrogen widely used in the 40s to 70s of past century to treat threatened miscarriage. Some years after DES started to be used, it was reported that exposed women had a high risk of breast cancer, their daughters had fertility problems, neoplasms, and autoimmune diseases, and their sons also had some less severe abnormalities.40,41 This led to develop experimental models to analyze the effects of DES as a potential ED. Neonatal exposure of CD-1 mice to doses of 1μg/kg/day was not associated to body weight changes during treatment, but was related to increased weight in adult age.42 Administration of high DES doses, 1000μg/kg/day, at that stage of development causes a significant weight decrease during treatment, while weight increase is subsequently seen in puberty. Weight increase in these rodents is due to an increased fat deposit, and is preceded by increases in levels of several adipokines such as leptin, adiponectin, IL-6, and triglycerides.43 On the other hand, prenatal administration at doses of 10–100μg/kg/day causes low birth weight, which is maintained throughout the life of the animal.
It has been speculated that early exposure to the endocrine disruptor would alter genetic programming of adipocytes and their distribution.43 The antiadipogenic or proadipogenic effect of DES exposure depends on exposure stage and dose.34 Molecular targets of estrogenic xenobiotics are estrogen receptors (ERs), mainly type α, mitochondria, and glucose transport in the cell.44
GenisteinGenistein is an isoflavone with antioxidant properties that has been used as an anthelmintic. Contact of genistein with the population mainly occurs through the intake of food products containing soya, which has recently experienced a boom in Western diet as additive or ingredient. Genistein has a weak estrogen activity and a chemical structure similar to estradiol, the main natural estrogen in humans.
Current evidence for the potential obesogenic activity of genistein was found by the Penza et al. group44 in 4-week C57BL/6 mice, in which pharmacological genistein doses inhibited far deposition, while at much lower than pharmacological doses it induced adipogenesis in the same experimental animals. Authors concluded that ginestein was adipogenic in immature mice at doses similar to those found in Western and Eastern diets containing soya. This effect has been shown to be due to overexpression of adipogenic genes and to be mediated by disruptor binding to estrogen receptors (ERs). This adipogenic effect is due to interaction with ERα, because it was not seen in αOKER mice.45 Ginestein binds to both ER α and ER β, showing preferential binding to these nuclear receptors, although it should not be forgotten that ER β receptors regulate ER α expression.46 Further studies are needed to establish the relative contribution of each of the above receptors.
The estrogenic effect of ginestein has been shown to depend on factors such as its concentration, endogenous estrogen levels, and animal gender.
High ginestein doses stimulate peroxisome proliferator-activated receptors (PPAR), preferentially PPARγ,47 inducing adipogenesis. In addition, in in vivo studies with cells from ovariectomized mice48 and in postmenopausal women,49,50 phytoestrogens decrease and modify body fat distribution.
Overall, it may be assumed that because of widespread exposure to ginestein of the general population and the adipogenic properties of this substance, shown in in vitro and in vivo animal studies, this xenobiotic is a good candidate obesogen in humans.
Bisphenol ABisphenol A (RPA) and nonylphenols are ubiquitous for the human population because of its widespread use in industrial and consumer products. They are also components of plastics used for food packaging. Their estrogenic effect is known since the 30s, and their levels in human serum range from 0.3 to 4.4ng/mL.30,51 In preadipocyte cultures (3T3-L1 cells), BPA, in the presence of insulin, activates adipogenic genes and promote cell differentiation, an effect mediated by its action on ERs.52,53In vivo, prenatal and exposure to BPA causes in mice obesity and hyperlipidemia associated to increased intake and decreased physical activity.53
In human adipocyte cultures, BPA has been seen to inhibit the release of adiponectin, which is known to protect the body against many of the components of the so-called metabolic syndrome.54 Additional evidence comes from an epidemiological study in humans where a correlation was seen between serum BPA levels, prevalence of obesity, and polycystic ovary syndrome.55 A positive association has also been seen between urinary BPA levels and BMI.56
All these data support the obesogenic activity of BPA in humans. However, a recent study by Teeguarden et al.57 showed that, in people who eat BPA-rich packaged food, BPA is rapidly absorbed by and eliminated from the body, which suggests that it would not have the toxic effect seen in experimental animals.
Organotin compoundsTributyltin (TBT), monobutyltin (MBT), and triphenyltin (TPT) are organic agents constantly polluting the environment. They are used in boat coating, in the wood industry, for water systems, and as fungicides in food.58,59 Organotin levels have been measured in human tissue samples in Europe and Asia. In liver samples, such levels range from 6 to 84ng/g body weight for MDT and are less than 2ng/g body weight for TBT.60 Blood organotin levels measured in the US are approximately 21ng/mL for the whole group of compounds and approximately 8ng/mL for TBT.61
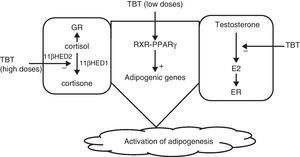
Grün and Blumberg36 suggested that disruptors would act by activating nuclear receptors such as PPAR and RXR, particularly PPARγ, which plays a significant role in adipogenesis. They could also act by inhibiting the aromatase that converts testosterone into estrogens, promoting adipogenesis, and finally, at high doses TBT would act upon cysteine residues to inhibit the action of 11β-hydroxysteroid dehydrogenase, increasing levels of cortisol, which also promotes adipogenesis62 (Fig. 2).
Organotin compounds induce maturation of adipocytes in culture, acting upon the RXR-PPARγ heterodimer.63–65 In mesenchymal stem cell cultures, they also induce formation of mature adipocytes after activation of the RXR-PPARγ heterodimer.65
Based on the above data and because of their extensive and frequent contact with a significant part of the general population, organotin compounds are potential obesogens in humans. Epidemiological studies showing an association between exposure to organotin compounds and obesity development are needed.
PhthalatesPhthalates are synthetic organic compounds derived from phthalic acid such as di-2-ethylhexylphthalate (DEHP) and mono-2-ethylhexylphthalate (MEHP). The general population is widely and continuously exposed to phthalates because they are components of plastics, cosmetic products, toys, lubricants, and so on. In humans, phthalate transfer in food processing has been reported to be approximately 160μg/day.66
Prenatal exposure of mice to DEHP doses higher than 5mg/kg body weight induces an antiadipogenic effect, acting through activation of PPARα.67,68 By contrast, chronic exposure to this same dose induces adipogenesis through activation of PPARγ.69 In preadipocyte cultures (3T3-L1), MEHP exposure induces adipogenesis by acting upon PPARγ.70,71 Prenatal phthalate exposure inhibits androgen synthesis and has an adipogenic effect.71 Finally, an epidemiological study in male human subjects reported a clear positive correlation between urinary phthalate levels and waist circumference.72
ConclusionsAmong xenobiotics considered as potential obesogenic compounds in humans, diethylstilbestrol, bisphenol A, organotin compounds, genistein, and phthalates have mainly been studied. Evidence for these agents comes from studies conducted in tissues and experimental animals, but very few data allowing to definitely establish their role as obesogens are available from studies in humans. Epidemiological studies showing the causative relationship between concentrations of the different obesogens in human biological fluids and obesity development are needed.
Conflict of interestThe authors state that they have no conflicts of interest.
Please cite this article as: García-Mayor RV, et al. Disruptores endocrinos y obesidad: obesógenos. Endocrinol Nutr. 2012;59:261–7.