Thyroid hormone resistance (THR) is a rare genetic syndrome in which tissue sensitivity to thyroid hormones is decreased, determining high levels of serum thyroid hormones and normal or elevated thyroid stimulating hormone (TSH).1 In most cases THR is due to mutations in the thyroid hormone receptor beta (THRB) gene.2
Suspicion of THR arises in the setting of inappropriate TSH secretion, where a TSH-secreting pituitary adenoma should also be ruled out. THR and TSH-omas have been classically considered two distinct entities, but differential diagnosis may be sometimes blurred.3
Thyroid autoimmunity may exist in THR,4 but reports have generally depicted a mild clinical picture. Case records describing the development of overt Graves’ disease with severe cardiac alterations are extremely rare.
A 46 year-old woman, with a previous history of simple hysterectomy due to uterine fibroids, was referred for evaluation of the following thyroid hormone profile: TSH 2.86μU/mL (normal 0.3–5.6), FT4 2.87ng/dL (0.8–1.7). She only recalled occasional increased heart rate and headache. Physical examination was unremarkable, except for a subtle goiter (grade Ia).
Repeated laboratory evaluation one month later revealed TSH 10.5, FT4 2.2, FT3 7.32pg/mL (2.5–3.9), anti-thyroperoxidase antibodies (TPOAb) 6170U/mL (<40), anti-thyroglobulin antibodies (TGAb) 126U/mL (<80) and anti-TSH-receptor antibodies (TSI) 11U/mL (<13). Pituitary laboratory evaluation showed IGF-1 142ng/mL (normal for age and sex), prolactin (PRL) 2541μU/mL (100–410), LH 1.2U/L, FSH 3U/L, estradiol 81pg/mL, ACTH 22pg/mL (9–54), cortisol 234ng/mL (65–230). Radioactive iodine uptake (RAIU) was not elevated. Pituitary magnetic resonance imaging (MRI) showed mild pituitary enlargement, with a hypoenhancing 13mm lesion on the left side. Levels of TRH-stimulated TSH, measured in a standard 90min test, increased more than 200% and levels of alpha-subunit were normal (0.31mU/mL, normal <1.6, ratio αTSH/TSH=0.05, normal<1). An octreotide suppression test showed no changes in thyroid hormones or PRL. Cabergoline 1mg/week was started, and titrated up to 2mg/week. Hyperprolactinemia was adequately controlled six months later, while biochemical hyperthyroidism persisted (TSH 5, FT4 2.5, PRL 401), and pituitary MRI showed reduction of the adenoma to 7mm.
Genetic testing identified a point mutation in the THRB gene (c.1642C>G) leading to a missense change of Proline 453 to Arginine (p.P453R). During the following months, the patient maintained clinical stability with TSH levels in the upper limit of normal and mildly elevated FT4 and FT3. PRL levels remained normal under cabergoline. The pituitary adenoma remained unchanged.
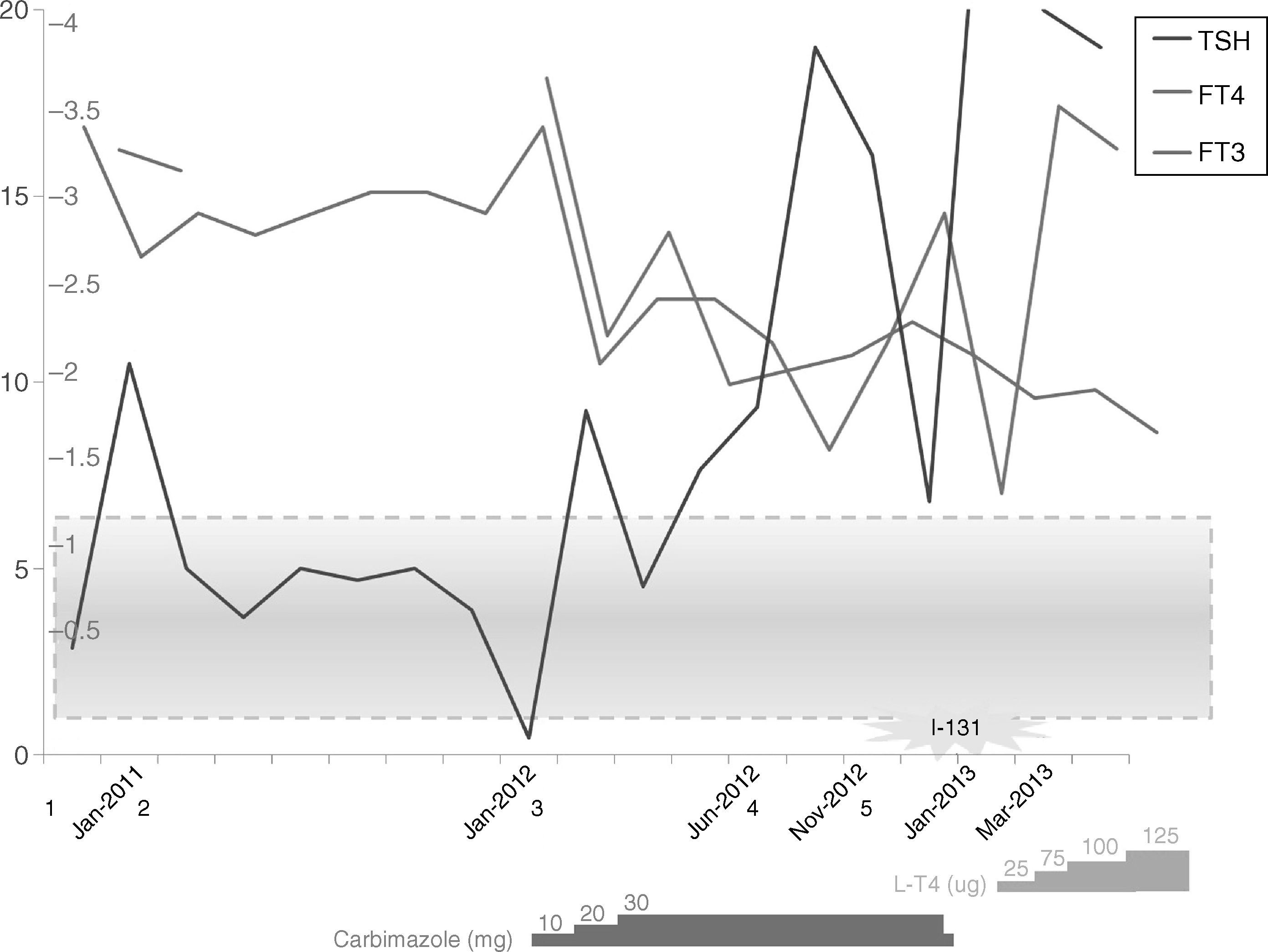
One year later, the patient developed sleeping difficulties, tremor, and anxiety. Physical examination of her thyroid had not change, but laboratory work up at this time revealed TSH 0.45, FT4 2.91, FT3 8.16, Anti-TPO 2572, Anti-TG 443, Anti-R-TSH 5, and RAIU showed a slightly increased uptake, suggesting the onset of primary hyperthyroidism. She started carbimazole 10mg/day, but, despite correct adherence to treatment and dose increase, euthyroidism was not achieved (Fig. 1), and she developed atrial fibrillation. I-131 therapy (16mCi) was performed. Laboratory follow-up showed progressive increase of TSH (up to 40μU/mL), with FT4 and FT3 in the normal-high range. She started low-dose L-T4 (25μg/day), which was then cautiously increased. At the last follow-up, her thyroid hormone levels were still elevated (TSH 20.0, FT4 3.3, FT3 4.0), but she remained asymptomatic, and with an adequate heart rate.
Timeline summarizing the main events the patient underwent. 1. Initial evaluation. 2. Genetic test results confirm syndrome of thyroid hormone resistance. 3. Onset of symptoms of hyperthyroidism. 4. Development of atrial fibrillation. 5. Cardioversion is performed. I-131: ablative treatment with I131 is performed. Values are shown as TSH μU/mL, FT4 ng/dL, FT3 pg/mL. Shaded blue box represents normal reference values.
Most cases of THR occur due to mutations in the THRB gene, but its associated phenotype may vary,1 and the gene defect may remain unknown in 15% of subjects.2 Suspicion of THR arises in the setting of inappropriate TSH production, but its diagnosis may be hindered if pituitary enlargement coexists,3 or by pitfalls in laboratory findings.5 In our patient, although the lack of family history could favor the existence of a TSH-secreting tumor, the null response to the octreotide test, the elevated levels of PRL, and the mild clinical picture despite high thyroid hormone levels, supported the hypothesis of THR and prolactinoma coexistence. THR was confirmed with a positive genetic testing, whilst the significant response to cabergoline suggested the existence of a prolactinoma.
THR and TSH-secreting pituitary tumors have been classically considered two different entities.3 Although long-standing absence of negative feedback has been suggested to determine enlargement of thyrotroph cells, the etiology of TSH-omas is not fully understood.5 Furthermore, given that T3 down-regulates transcription of the human PRL gene in pituitary cells through T3-responsive elements in its promoter, TRβ mutations may lead to hyperprolactinemia, determining pituitary hyperplasia and adenoma development. However, to our knowledge, hyperprolactinemia and prolactinomas have not been specifically addressed in the setting of THR. We could hypothesize that mutations in certain TRβ isoforms could lead to the development of pituitary tumors, so THR and pituitary adenomas would coexist as a result of the same physiopathogenic mechanism, and would not be two independent alterations.
Another distinctive feature of our patient is that she developed a marked autoimmune primary hyperthyroidism one year later. Autoimmune thyroid disease has been documented in cases of THR,4 but in the majority of publications, either THR coexisted with Hashimoto's disease, or suspicion of THR arose after an initial diagnosis of Graves’ disease.6,7 However, to our knowledge, only two reports have described the development of Graves’ disease in patients with a known and stable THR.8,9 The natural history explaining this outcome is not clear.
THR and autoimmunity may merely coexist, given the high prevalence of thyroid autoimmunity in general, but other reports suggest that autoimmunity develops following persistent stimulation of thyroid lymphocytes, and assert that these entities are truly associated, and not merely coincidental.4 It has been proposed that the immune system may be activated by another mechanism in THR, for instance via either the TRβ or the TRα,4 in a similar way as in the development of tachycardia.2
Favoring the latter hypothesis, our patient already presented thyroid autoimmunity at the first evaluation. However, she subsequently developed hyperthyroidism and cardiac alterations, which, although relatively frequent in Graves’ disease, are less common in THR.10 The reason for such a clinical course of hyperthyroidism in our patient is not fully elucidated. Maybe THR delayed an overt hyperthyroid spectrum, but once hyperthyroidism developed, it outweighed peripheral resistance. Future research deems necessary to investigate the autoimmune environment in individuals with THR.
In conclusion, we remark the difficulties encountered in distinguishing the cause of inappropriate TSH secretion when a pituitary adenoma coexists. These two entities may comprise the phenotypic expression of a unique genetic alteration. Thyroid autoimmunity may coexist with THR, or subsequently develop due to chronic TSH stimulation. The extent to which this may influence progression to severe Graves’ disease, like in our patient, remains to be clarified. Ablative treatment may be necessary in cases of persistent hyperthyroidism that outweighs peripheral resistance.
Author contribution statementARL followed the patient, analyzed and interpreted data, and wrote, reviewed and edited the manuscript. JM performed molecular studies and revised the manuscript. NL performed molecular studies. CAE followed the patient and contributed to interpretation of the clinical setting. MM followed the patient and revised and edited the manuscript. All authors contributed to the final version of this manuscript.
Conflict of interestThe authors declare no conflict of interest.