To assess the long-term clinical and economic impact of integrated pump/CGM technology therapy as compared to multiple daily injections (MDI), for the treatment of type 1 diabetes (T1D) in Colombia.
MethodsThe CORE Diabetes Model was used to simulate a hypothetical cohort of patients with T1D. Mean baseline characteristics were taken from a clinical study conducted in Colombia and a healthcare payer perspective was adopted, with a 5% annual discount rate applied to both costs and outcomes.
ResultsThe integrated pump/CGM improved mean life expectancy by 3.51 years compared with MDI. A similar increase occurred in mean quality-adjusted life expectancy with an additional 3.81 quality-adjusted life years (QALYs). Onset of diabetes-related complications was also delayed as compared to MDI, and mean survival time free of complication increased by 1.74 years with integrated pump/CGM. Although this increased treatment costs of diabetes as compared to MDI, savings were achieved thanks to reduced expenditure on diabetes-related complications. The estimated incremental cost-effectiveness ratio (ICER) for SAP was Colombian Pesos (COP) 44,893,950 (approximately USD$23,200) per QALY gained.
ConclusionsImproved blood glucose control associated to integrated pump/CGM results in a decreased incidence of diabetes-related complications and improves life expectancy as compared to MDI. Using recommended thresholds from the World Health Organization and previous coverage decisions about health technologies in Colombia, it is a cost-effective alternative to MDI for the treatment of type 1 diabetes in Colombia.
Evaluar los impactos clínicos y económicos de largo plazo de la terapia con bomba de insulina integrada a sistema de monitorización continua de glucosa (MCG) vs. inyecciones múltiples diarias de insulina (MDI) en pacientes con diabetes tipo 1 (DT1) en Colombia.
MétodosSe usó el CORE Diabetes Model con el fin de simular una cohorte hipotética de pacientes con DT1. Las características promedio de línea base fueron tomadas de un estudio clínico local. La perspectiva fue desde del pagador y se aplicó una tasa de descuento del 5% para los costes y los resultados.
ResultadosLa bomba de insulina integrada al sistema de MCG mejoró la expectativa de vida media en 3,51 años y 3,81 años de vida ajustados por calidad adicionales. En comparación con MDI hubo un retraso en el inicio de complicaciones relacionadas con la DT1, y el tiempo promedio de sobrevida y libre de cualquier complicación se aumentó en 1,74 años. Los costes relacionados con la bomba de insulina integrada al sistema de MCG fueron compensados con los ahorros por la disminución en las complicaciones relacionadas con la DT1. La relación de coste efectividad incremental fue de 23.200 dólares americanos por años de vida ajustados por calidad.
ConclusionesEl mejor control glucémico asociado con bomba de insulina integrada al sistema de MCG conduce a una disminución en la incidencia de las complicaciones relacionadas con DT1 y aumenta la esperanza de vida cuando se compara con MDI. De acuerdo a los umbrales recomendados por la Organización Mundial de la Salud, esta es una estrategia coste-efectiva cuando se compara con MDI en el tratamiento de la DT1.
Diabetes mellitus (DM) is a significant health problem in Colombia and a growing challenge for its health system. DM prevalence is 9.6% (8.6–10.7%) in the population aged 20–79 years, and it is estimated that one out of 1000 children (0–14 years) has type 1 diabetes mellitus (T1DM).1 In addition, Aschner and Aguilar reported an 8.0% prevalence of DM in South and Central America, and children with T1DM account for 0.2% of the total population with DM.2 In Colombia, Aschner found a prevalence ranging from 4% to 8% depending on the age range of the study population, and estimated at 0.07% the prevalence of T1DM in children under 15 years of age.3
Barcelo et al. estimated the total economic costs of T1DM and type 2 diabetes mellitus (T2DM) to be US$ 2.5 billion for 2003. This was a relatively low amount as compared to other Latin American countries.4 Barcelo et al., in The cost of diabetes in Latin America and the Caribbean. Bulletin of the World Health Organization. 2003, attributed this low economic burden of the disease to the low frequency of the use of outpatient clinics (0.7%) and hospitalization services (6.1%) by the Colombian population.4
T1DM is associated with an increased risk of severe complications, and associated cardiovascular disease is the attributable cause of death in 47% of men and 41% of women with T1DM.5 Beyond the clinical impact, complications related to T1DM have significant implications for patient quality of life (QoL). Solli et al. evaluated the results of the EuroQol 5-Dimensions (EQ-5D) questionnaire, which allows for assessing the quality of life of a given state of health between 0 and 1, where 0 represents death and 1 a full state of health. Researchers found that patients with T1DM had, on a 0 to 1 scale, a mean value of 0.9 if they were free of complications, and a value of 0.68 if there were complications related to T1DM.6 After assessing the main determinants of decreased QoL within the EQ-5D subdomains, the authors found “fear of hypoglycemia” to be a significant determinant of “anxiety/depression”, one of the five categories of the questionnaire.
Complications related to T1DM also have a significant impact on health care expenses, and costs related to complications usually exceed those directly attributed to treatment. Optimizing the management of blood glucose levels through effective self-monitoring has been shown to decrease the risk of developing the related complications. The effective treatment of T1DM is usually cost-effective.7 Despite the benefits of the self-monitoring of blood glucose (SMBG) in T1DM, the evidence shows that up to 63% of patients do not perform SMBG regularly.8 This may be due to the complexity of the associated time requirements. In a review of the literature on adherence to the different treatment regimens for T1DM, Delamater et al. reported that patients had a better adherence to simple as compared to more complex regimens, and that lower SMBG rates were seen in Hispanic patients as compared to the general population.9
Continuous glucose monitoring (CGM) may represent a significant advance, because it is easier to use and promotes adherence. A randomized, controlled clinical study comparing CGM and SMBG found a greater adherence to CGM, which was used six or more days per week by 83% of patients aged 25 years or older.10 The advantages of CGM over SMBG and multiple daily insulin (MDI) injections have been shown in several studies.10–16 Unfortunately, hypoglycemic events may occur even with CGM, and the number of these events is often underestimated. In 2013, Ly et al. showed that an automated insulin injection system that interrupts injection for up to two hours if CGM detects a glucose level less than 60mg/dL significantly decreases the rate of symptomatic and severe hypoglycemic episodes.14 Bergenstal et al. found that nighttime hypoglycemic events were 31.8% less common in the group with an interruption threshold as compared to the control group (1.5±1.0 vs 2.2±1.3 by patient and week, p<0.001).12 This integrated pump/CGM technology therapy has the potential to decrease glycosylated hemoglobin (HbA1c) levels and the frequency of hypoglycemic events as compared to MDI.10,12–15
By evaluating the current and future economic burden of T1DM for the Colombian health system, the impact of future treatments may be estimated. Such evaluations help decision makers of the health system in the resource optimization process.17 This analysis assesses the clinical and economic impact of integrated pump/CGM technology therapy as compared to MDI.
MethodsModelFor the cost-effectiveness analysis, a Markov model allowed for the long-term projection of clinical results, including associated complications, and direct medical costs. The IMS CORE Diabetes Model (CDM) version 8.5, developed by imshealth, was used because of its high validation level and its wide use in many countries worldwide.
CDM is a model used to simulate the progression of diabetes and its associated results.18,19 It is constructed as a Markov model with several submodels run in parallel to simulate the incidence of the complications. The foregoing is based on HbA1c progression and treatment schemes. In this analysis, a second-order Monte Carlo simulation is performed with 1000 patients and 1000 iterations. Myocardial infarction and stroke risk equations were derived from the United Kingdom Prospective Diabetes Study, while equations from the Framingham study were used to estimate the risks of angina and heart failure.
The development of complications (cumulative incidence and time to occurrence), quality-adjusted life expectancy measured as quality-adjusted life years (QALYs), direct medical costs, and indirect costs were modeled for each of the treatment options.18
Clinical benefitsIn order to parametrize the clinical impact of integrated pump/CGM technology therapy in Colombia, data were taken from the Gómez et al. Study.20 This study reported a significant mean HbA1c reduction of 1.5% (16mmol/mol) in the 217 study patients. The authors also reported a significant decrease in the annual rate of severe hypoglycemia from 5.22 to 0.37 episodes per year (p=0.0009).
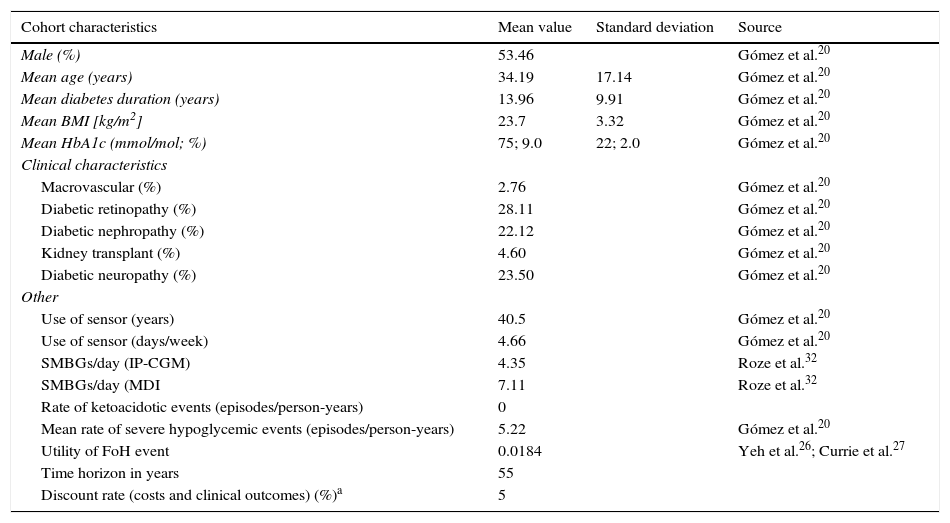
CohortThe baseline characteristics of the cohort for analysis were taken from the recent clinical study by Gómez et al., and may be found in Table 1.20 Although the average use of the sensor was not reported, the value calculated from the available data was 66.6, equivalent to 4.66 days-sensor per week. The relative frequency of SMBG was taken from an observational study conducted by Personss et al. in which the use of SMBG was reported to be lower with integrated pump/CGM technology therapy as compared to MDI.21
Characteristics of the population.
| Cohort characteristics | Mean value | Standard deviation | Source |
|---|---|---|---|
| Male (%) | 53.46 | Gómez et al.20 | |
| Mean age (years) | 34.19 | 17.14 | Gómez et al.20 |
| Mean diabetes duration (years) | 13.96 | 9.91 | Gómez et al.20 |
| Mean BMI [kg/m2] | 23.7 | 3.32 | Gómez et al.20 |
| Mean HbA1c (mmol/mol; %) | 75; 9.0 | 22; 2.0 | Gómez et al.20 |
| Clinical characteristics | |||
| Macrovascular (%) | 2.76 | Gómez et al.20 | |
| Diabetic retinopathy (%) | 28.11 | Gómez et al.20 | |
| Diabetic nephropathy (%) | 22.12 | Gómez et al.20 | |
| Kidney transplant (%) | 4.60 | Gómez et al.20 | |
| Diabetic neuropathy (%) | 23.50 | Gómez et al.20 | |
| Other | |||
| Use of sensor (years) | 40.5 | Gómez et al.20 | |
| Use of sensor (days/week) | 4.66 | Gómez et al.20 | |
| SMBGs/day (IP-CGM) | 4.35 | Roze et al.32 | |
| SMBGs/day (MDI | 7.11 | Roze et al.32 | |
| Rate of ketoacidotic events (episodes/person-years) | 0 | ||
| Mean rate of severe hypoglycemic events (episodes/person-years) | 5.22 | Gómez et al.20 | |
| Utility of FoH event | 0.0184 | Yeh et al.26; Currie et al.27 | |
| Time horizon in years | 55 | ||
| Discount rate (costs and clinical outcomes) (%)a | 5 | ||
IP-CGM: integrated pump/CGM technology therapy; MDI: multiple dose insulin injections.
The discount rate is taken from the Colombian Methodological guide for conducting economic evaluations in the setting of clinical practice guidelines, version 10/03/2014 [accessed 9 Jan 2015]. Available at: www.iets.org.co.
The study looks at the direct costs of T1DM from the payer's perspective, calculated in 2013 Colombian pesos and converted to US dollars to facilitate international comparison using the rate of exchange applicable on 31 December 2013, which was 1869.16 Colombian pesos to the US dollar. The total costs correspond to treatment of diabetes and its complications. These costs were calculated on the basis of the official tariff manual for Colombia (SOAT) and the billing data of public and private payers. The costs of intervention for integrated pump/CGM technology therapy were defined on the basis of the selling price of the Enlite® Sensor device, the Enlite® Specific Serter, the MinilinkTM transmitter, batteries, lancets, and strips (pack of 50) provided by the manufacturer. A total of 40.5 Enlite® Sensors were used for the base case. The intervention costs of daily insulin injections included the costs of lancets and strips.
UtilitiesThe different utility values of the health states were taken from several previously published sources,22–25 and when multiple utility values had to be applied at the same time, the state with the lowest value was selected. If a patient experienced myocardial infarction, stroke, amputations, or hypoglycemic episodes, utility values were discounted from total QALYs.
Yeh et al. reported that CGM decreased the fear of hypoglycemia (FoH) as compared to SMBG.26 In their study, mean difference between the groups with a fear of hypoglycemia was –2.3 (95% CI –8.2 to 3.6) in favor of CGM. In terms of utility, a decrease in EQ-5D by 0.008 units per unit increase in FoH in the fear of hypoglycemia survey has been reported.27 The utility of FoH applied in this study was +0.0184 (2.3×0.008) in patients with integrated pump/CGM technology therapy, and reflects the benefits derived from FoH decrease.
Discount rateAs recommended for Colombia, a 5% discount rate was applied to both the clinical and economic results (Table 1). In order to ensure that all impacts in terms of health outcomes and the costs of treatment alternatives were included in the model, a lifetime analysis horizon was assumed.
Sensitivity analysisTo assess the robustness of the model and construct acceptability curves of willingness to pay, a probabilistic sensitivity analysis was performed with sampling of distributions describing the uncertainties in the parameters of the variables.
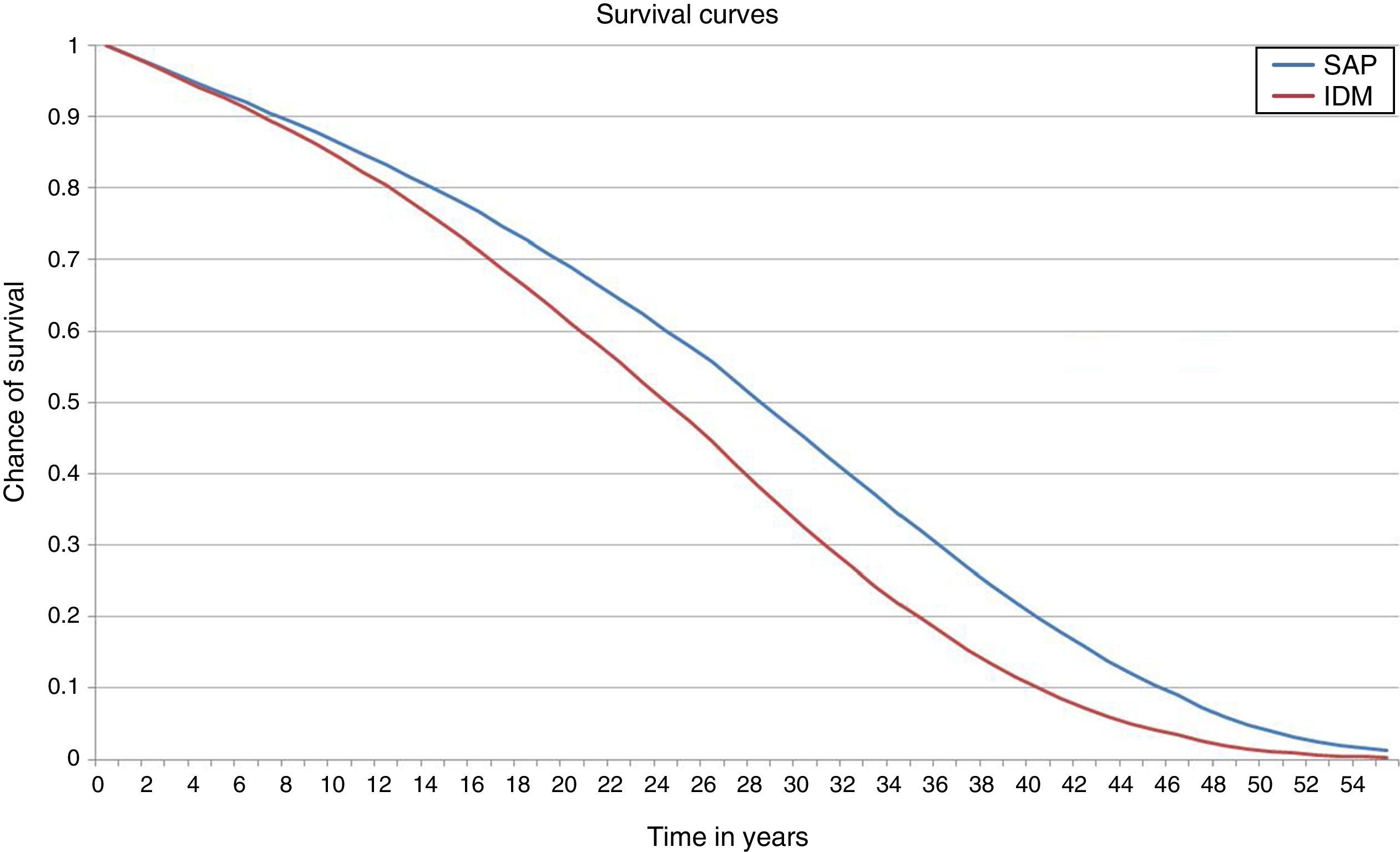
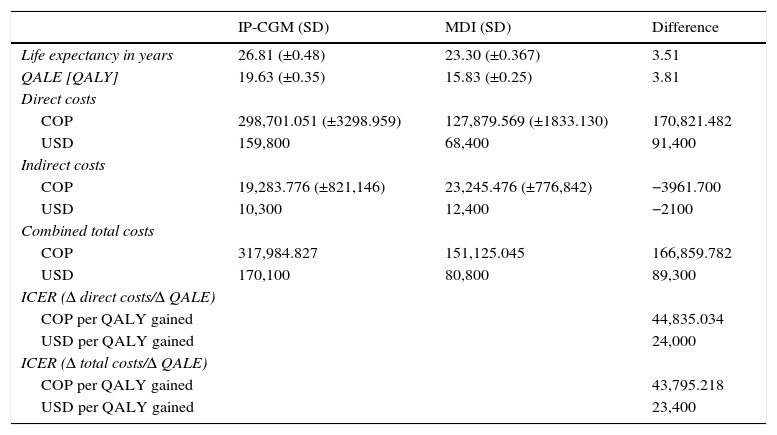
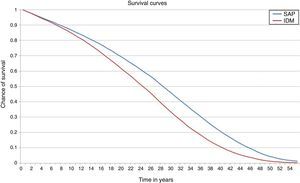
ResultsIn the base case analysis, the treatment of T1DM with integrated pump/CGM technology therapy increased life expectancy by 3.52 years (95% CI: 3.47–3.55) as compared to MDI (Fig. 1 and Table 2). Mean life expectancy in the group with integrated pump/CGM technology therapy was 26.81±0.48 years, as compared to 23.30±0.38 years in the MDI group. In addition, the group with integrated pump/CGM technology therapy achieved 19.63±0.35 QALYs, as compared to 15.83±0.25 QALYs in the MDI group, i.e. a difference of 3.81 QALYs.
Results of base case.
| IP-CGM (SD) | MDI (SD) | Difference | |
|---|---|---|---|
| Life expectancy in years | 26.81 (±0.48) | 23.30 (±0.367) | 3.51 |
| QALE [QALY] | 19.63 (±0.35) | 15.83 (±0.25) | 3.81 |
| Direct costs | |||
| COP | 298,701.051 (±3298.959) | 127,879.569 (±1833.130) | 170,821.482 |
| USD | 159,800 | 68,400 | 91,400 |
| Indirect costs | |||
| COP | 19,283.776 (±821,146) | 23,245.476 (±776,842) | −3961.700 |
| USD | 10,300 | 12,400 | −2100 |
| Combined total costs | |||
| COP | 317,984.827 | 151,125.045 | 166,859.782 |
| USD | 170,100 | 80,800 | 89,300 |
| ICER (Δ direct costs/Δ QALE) | |||
| COP per QALY gained | 44,835.034 | ||
| USD per QALY gained | 24,000 | ||
| ICER (Δ total costs/Δ QALE) | |||
| COP per QALY gained | 43,795.218 | ||
| USD per QALY gained | 23,400 | ||
IP-CGM: integrated pump/CGM technology therapy; MDI: multiple dose insulin injections.
Conversion from Colombian pesos (COP) to US dollars (USD) (rounded to the nearest %) at an exchange rate of 1 COP per 0.000535 USD.
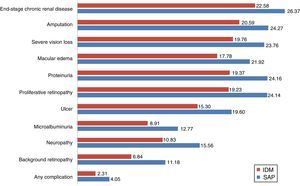
Considering the complications related to T1DM, integrated pump/CGM technology therapy delayed the mean time to the occurrence of the first complication by approximately 1.74 years. The mean time free of complications was 4.05 years, as compared to 2.38 years with MDI (Fig. 2).
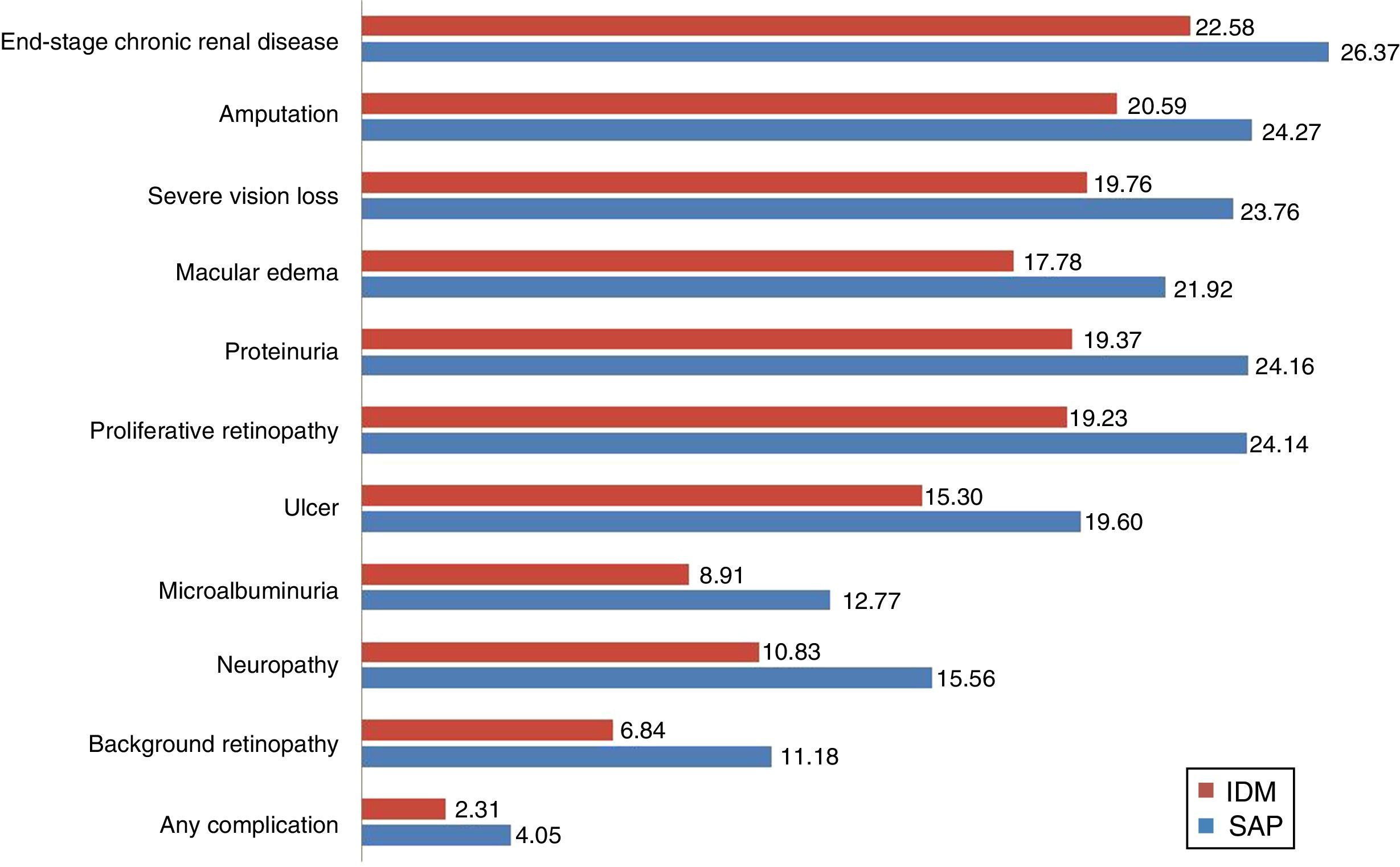
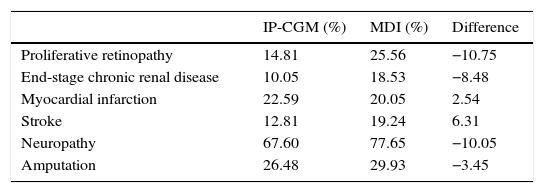
The group with integrated pump/CGM technology therapy had a mean delay of 4.5 years in the start of three complications: neuropathy (4.73 years), proteinuria (4.79 years), and proliferative retinopathy (4.91 years). In addition to the delay in the start of complications, the cumulative lifetime incidence of complications related to T1DM was lower as compared to the MDI group, and the cumulative incidence of end-stage renal disease was 10.1%, as compared to 18.5% for MDI (Table 3). However, because of increased life expectancy, the group with integrated pump/CGM technology therapy had a greater cumulative incidence of some complications related to older age as compared to the MDI group (the so-called “survival paradox”), as shown in Table 3.
Cumulative incidence of complications related to T1DM.
| IP-CGM (%) | MDI (%) | Difference | |
|---|---|---|---|
| Proliferative retinopathy | 14.81 | 25.56 | −10.75 |
| End-stage chronic renal disease | 10.05 | 18.53 | −8.48 |
| Myocardial infarction | 22.59 | 20.05 | 2.54 |
| Stroke | 12.81 | 19.24 | 6.31 |
| Neuropathy | 67.60 | 77.65 | −10.05 |
| Amputation | 26.48 | 29.93 | −3.45 |
IP-CGM: integrated pump/CGM technology therapy; MDI: multiple dose insulin injections.
The mean direct costs associated with integrated pump/CGM technology therapy were projected at US$ 159,800, as compared to US$ 68,400 for MDI (Table 2). The incremental cost-effectiveness ratio (ICER) was US$ 24,000. Assuming a willingness to pay of US$ 26,750 (approximately 3 times GDP per capita) per QALY gained,28 the acceptability to pay curve showed that integrated pump/CGM technology therapy was cost-effective in 99% of cases.
The evaluation of components of direct costs showed that the main determinants were the costs of the treatments. Thus, integrated pump/CGM technology therapy and MDI accounted for 86% and 61% of direct costs respectively. The lifetime costs of treatment were US$ 137,867 for integrated pump/CGM technology therapy and US$ 41,847 for MDI. The longer life expectancy associated with integrated pump/CGM technology therapy accounted for a 13% increase in costs. The remaining direct costs were related to the management and treatment of complications related to T1DM. Substantial differences were found between the two groups regarding the cost of the treatment of complications. Although the most important components of the cost of complications in both hypothetical cohorts were attributed to the treatment of ulcers, amputations, and neuropathy, this accounted for 6% of direct costs with integrated pump/CGM technology therapy and for 18% of direct costs in the MDI group. On average, the cost of treating complications was lower as compared to MDI (US$ 20,987 vs US$ 25,638), with cost decrease attributed to a lower cumulative incidence of complications.
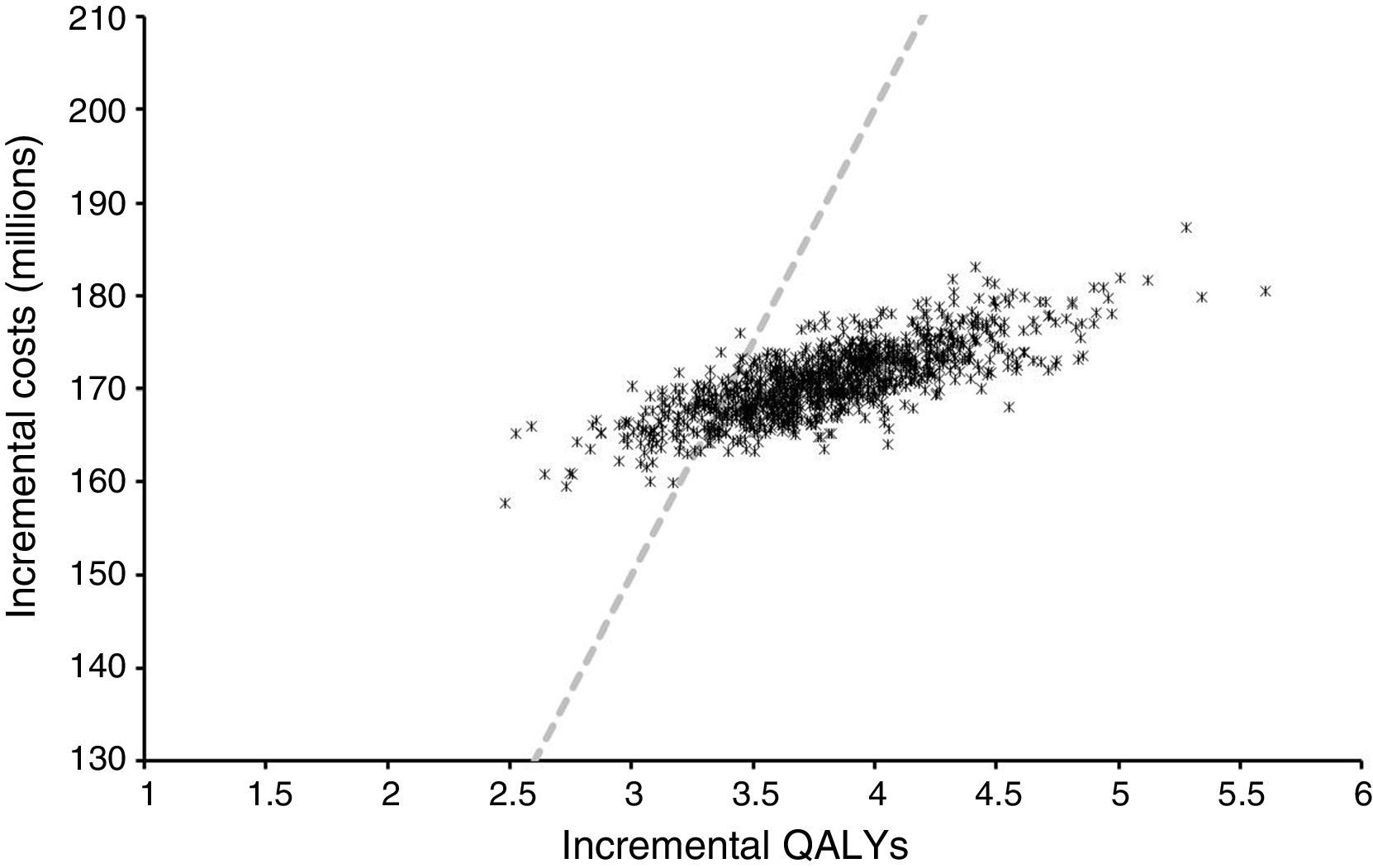
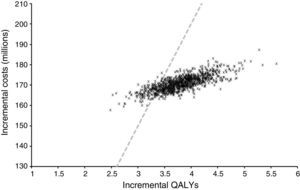
Sensitivity analysisProbabilistic sensitivity analysis allowed us to explore changes in outcomes due to uncertainty in the model and its parameters. Fig. 3 shows the distribution of the incremental cost and QALYs of integrated pump/CGM technology therapy as compared to MDI from 1000 simulations.
In all cases, this was associated with increased quality-adjusted life expectancy and to an increase in direct costs. The findings of this study may, therefore, be considered robust and interpreted with confidence. The mean ICER of these simulations was US$ 24,269 per QALY with 25th and 75th percentiles of US$ 22,680 and US$ 25,652 per QALY gained respectively. If indirect costs, those not related to medical costs but rather to the patient, were also to be taken into consideration, the ICER would increase. When estimated using direct and indirect costs, ICER amounted to US$ 23,400 per QALY.
DiscussionIn the long term, cost-effectiveness analyses suggest that treatment with integrated pump/CGM technology is associated with better clinical outcomes and a lower incidence of complications related to T1DM as compared to MDI. Integrated pump/CGM technology therapy is associated with higher direct medical costs, which are partially compensated for by a decreased complication rate. ICER suggests that integrated pump/CGM technology therapy is probably cost-effective as compared to MDI for the treatment of T1DM under Colombian conditions. According to the WHO recommendation of a willingness to pay with a threshold of 3 times GDP per capita,28,29 there was a 99% chance that integrated pump/CGM technology therapy would be considered cost-effective in Colombia. If indirect costs were also taken into consideration, this chance would increase.
Other cost-effectiveness analyses comparing the two alternatives for action and conducted in other settings have reached similar conclusions. In a comparison with standard SMBG management, McQueen et al. estimated that the use of CGM for the treatment of T1DM was cost-effective, with an ICER of US$ 45,033 per QALY gained.30 Similarly, Huang et al. estimated the ICER for CGM as compared to standard glucose monitoring in T1DM at US$ 78,943 per QALY gained.31 The ICER estimated in this study is favorable and may reflect the high baseline HbA1c, which provides an increased possibility of better results. In this context, the Pickup et al. meta-analysis showed that greater HbA1c reductions were associated with higher baseline values.15 In addition, the evaluation of the impact of integrated pump/CGM technology therapy on FoH may provide further benefits. Ly et al. showed that integrated pump/CGM technology therapy was very helpful in decreasing the rates at which patients with poor awareness of hypoglycemia experienced severe hypoglycemic episodes.14
The cost–effectiveness study conducted by Kamble et al.,33 published in 2012, found an ICER of US$ 168,104 per QALY gained with the insulin pump for a scenario of sensor use for six days, and concluded that this is not yet economically viable in the United States. Although this study used the CORE Diabetes Model, this is not made clear in the utilization of the costs of long-term complications of T1DM, and only costs related to the consumables and insulin required for treatment in each of the two arms, as well as hospitalization events and emergency visits directly related to diabetes, are mentioned. Estimates of effectiveness were also taken from data from the STAR 3 study, which were lower than the 1.2% decrease used by St. Charles et al.34 from a meta-analysis.
Special care was taken to ensure that this analysis was an accurate representation of the use of integrated pump/CGM technology therapy in the Colombian setting. However, as with all modeling studies in health economics, the analysis presented here provides estimates of the clinical and economic implications based on simulations from data reported and scenarios of the authors themselves. Because of the lack of long-term studies in T1DM, short-term data should be used to project results. In order to minimize the potential limitations of this study, an extensive, systematic search was made in the worldwide literature, and according to availability in the local setting. In our case, the unique demographic and epidemiological characteristics of the hypothetical cohorts were taken from specific literature on integrated pump/CGM technology therapy in Colombia.
A limitation of this study is that the CORE Diabetes Model is based on data from the United Kingdom Prospective Diabetes Study, the Diabetes Control and Complications Trial, the Framingham Heart Study, and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Although these epidemiological data are widely used for modeling the health outcomes of patients with diabetes, they are not always representative of the progression of complications in patients diagnosed with T1DM and treated more recently. However, validation studies have shown that simulations with CDM have provided statistical R2 values of 0.9. For validation studies with follow-up for T1DM, R2 values of 0.9 for periods shorter than 15 years and 0.72 for 30-year follow-up periods (DCCT) have been reported.35
FundingThis study was supported by Medtronic PLC.
Conflicts of interestAll the authors contributed to the research and preparation of the manuscript and meet authorship criteria. SR and RS are employees in the consulting company and received payment for their participation in the research. PML, JEV, and JO are employees of Medtronic PLC. RAC and AMG have received no honoraria for this study from Medtronic PLC. The authors declare their full independence throughout the research process and are solely responsible for the methods, results, and concepts contained in this manuscript.
Please cite this article as: Gomez AM, Alfonso-Cristancho R, Orozco JJ, Lynch PM, Prieto D, Saunders R, et al. Beneficios clínicos y económicos de la terapia con bomba de insulina integrada a sistema de monitoreo continuo de glucosa en los pacientes diabéticos tipo 1 en Colombia. Endocrinol Nutr. 2016;63:466–474.