Iodine deficiency affecting both pregnant women and schoolchildren has been reported in Jaén. Iodine deficiency is one of the leading causes of thyroid dysfunction and goiter, and adequate iodine prophylaxis with iodized salt, milk, and dairy products, or iodine supplementation have been shown to significantly improve iodine status in pregnancy. The purpose of this study was to assess iodine nutritional status in the general population of a iodine-deficient area with no previous institutional campaigns of iodine prophylaxis.
Material and methodsA descriptive, cross-sectional study. Urinary iodine levels were measured in subjects from the Jaén healthcare district. The data were stratified by sex and age groups, and a survey was conducted on iodized salt consumption.
ResultsMedian and mean urinary iodine levels were 110.59mcg/L and 130.11mcg/L respectively. Urinary iodine levels were significantly higher in schoolchildren as compared to other age groups (161.52μg/L vs 109.33μg/L in subjects older than 65 years). Forty-three percent of the population had urinary iodine levels less than 100μg/L, and 68% of women of childbearing age had levels less than 150μg/L.
ConclusionsIodine nutritional status appears to be adequate, but the proportion of the population with urinary iodine levels less than 100μg/L is still very high, and iodized salt consumption is much less common than recommended by the WHO.
En Jaén se conoce que existe una deficiencia de yodo (DY) de leve a moderada, y que afecta tanto a escolares como a mujeres embarazadas. Se sabe que la DY es una de las causas principales de disfunción tiroidea y bocio, habiéndose establecido que una yodoprofilaxis adecuada en zonas yododeficientes, tanto en forma de sal yodada, leche y sus derivados, o la toma de suplementos yodados, en caso de gestación, conlleva una mejoría significativa de estos problemas. El objetivo de este estudio es evaluar el grado de nutrición yódica en población general en una zona catalogada como yododeficiente y sin que se hayan llevado a cabo, por el momento, campañas institucionales de yodoprofilaxis.
Material y métodosEstudio descriptivo de corte transversal. Se ha realizado determinación de la yoduria en población general en el distrito sanitario de Jaén, separando en grupos según la edad y el género, y se ha encuestado sobre del consumo de sal yodada.
ResultadosLa mediana de yoduria fue de 110,59μg/l y la media de 130,11μg/l. Se encuentran diferencias estadísticamente significativas en los niveles de yoduria en los escolares con respecto al resto de grupos de edad, siendo la media de yoduria en este grupo de 161,52μg/l vs 109,33μg/l en los mayores de 65 años. Encontramos que el 43% de la población tiene una yoduria menor de 100μg/l y que en las mujeres, en el grupo de edad fértil, hay un 66,8% con niveles de yoduria inferior a 150μg/l.
Conclusionesla situación nutricional de yodo indicaría que se encuentra dentro de lo que se considera una nutrición adecuada, si bien encontramos que el porcentaje de población que presenta yodurias por debajo de 100μg/l es aún muy elevado, y que la prevalencia del consumo de sal yodada en hogares es del 30,9%, muy por debajo de las recomendaciones de la OMS.
Doctors F. Escobar del Rey and G. Morreale de Escobar promoted the study of iodine deficiency in Spain, with multiple reports demonstrating the magnitude of this problem.1–4
Over the last 20 years, many publications have reported changes over time in iodine nutrition in various Spanish regions. Studies conducted in schoolchildren,5 pregnant women,6 and the adult population7 have been particularly relevant. Some studies provide examples of cooperation between scientific bodies and health authorities, such as the establishment of iodine prophylaxis schemes in some regions, as occurred in Asturias.8,9
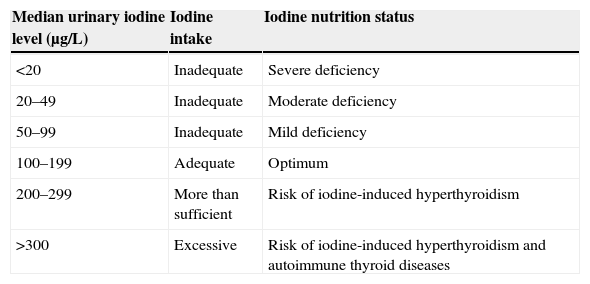
Understanding the situation in this regard is important because iodine deficiency (ID) is related, amongst other things, to the occurrence of changes in the psychomotor development of children. These range from minor problems with mild ID to endemic cretinism with highly severe ID.5,10,11 Goiter is the most typical manifestation of ID. And although women of childbearing age, infants, and schoolchildren are the most exposed subjects, the whole population can benefit from iodine prophylaxis campaigns aimed at improving iodine nutrition in society in general (Table 1).
Classification of nutrition status based on urinary iodine levels in schoolchildren using the ICCIDD, UNICEF, and WHO criteria.11
| Median urinary iodine level (μg/L) | Iodine intake | Iodine nutrition status |
|---|---|---|
| <20 | Inadequate | Severe deficiency |
| 20–49 | Inadequate | Moderate deficiency |
| 50–99 | Inadequate | Mild deficiency |
| 100–199 | Adequate | Optimum |
| 200–299 | More than sufficient | Risk of iodine-induced hyperthyroidism |
| >300 | Excessive | Risk of iodine-induced hyperthyroidism and autoimmune thyroid diseases |
UNICEF, ICCID (the International Council for Control of Iodine Deficiency), and the WHO12 have issued specific recommendations for iodine intake based on age, and proposed indicators of iodine nutritional status in the community (Table 2). Urinary iodine level is the most important indicator, because while thyroglobulin and neonatal TSH levels and the prevalence of goiter in schoolchildren reflect evolution over time to adequate iodine intake, urinary iodine is a more up-to-date and immediate measure of iodine nutrition status in any studied population because a fairly stable balance is established between iodine ingested and excreted.
Mean urinary iodine level (mcg/L) in the different age groups.
| <15 years*n=41♂ and 40♀ | 15–40 yearsn=121 ♂ and 226 ♀ | 40–65 yearsn=149 ♂ and 199 ♀ | >65 yearsn=114 ♂ and 113 ♀ |
|---|---|---|---|
| 161.52(CI: 140.98–182.05μg/L) | 145.37(CI: 134.80–155.93μg/L) | 125.01(CI: 117.45–132.54μg/L) | 109.33(CI: 96.75–121.50μg/L) |
They have also established an action protocol through the organizations involved in public health, recommending that the most adequate way to correct ID is the use of iodized salt. This protocol has been adapted both to general Spanish requirements13 and to the specific requirements of each Spanish region, although in Andalusia there has been no institutional campaign to assess iodine nutrition in the population, nor iodine prophylaxis campaigns to promote the use of iodized salt. Studies have shown, however, that mild to moderate ID exists in the province of Jaén.5,14
Our study had two main objectives: to ascertain iodine nutrition in the general population by measuring urinary iodine levels, and to determine the intake of iodized salt as iodine prophylaxis in this population.
Subjects and methodsA multicenter, cross-sectional, descriptive study was conducted in rural and urban health care areas. The estimated sample size was 1200 subjects, but only 1011 subjects were finally recruited. The main reason for having a sample size smaller than anticipated was that pediatricians rarely request laboratory tests for children under 15 years of age, and we thought it inappropriate to enroll them in the study and take a blood sample even when they met the inclusion criteria.
The study was conducted in subjects of both sexes and all ages and races who were receiving care in the Jaén health district (from 11 different health care centers covering 220,000 inhabitants of the province of Jaén), who were not hospitalized, who had demanded primary health care and for whom laboratory tests had been requested for a reason unrelated to a possible thyroid disease. Current or past thyroid or endocrine-metabolic conditions were ruled out, as well as other pathological processes whose nature might alter thyroid function measurements, such as chronic kidney disease, euthyroid sick syndrome, AIDS, amyloidosis, scleroderma, sarcoidosis, diseases of tumor origin, leukemia, and lymphoma. People who had required iodine-containing treatments such as disinfectants, iodinated contrast agents for imaging tests, amiodarone or other drugs altering thyroid function (bexarotenes, lithium, interferon, interleukin, phenobarbital, rifampin) were also excluded.
Subjects were selected by investigators with the logistic support of the managers of the health care centers which participated in the study between January 2013 and May 2014. All subjects who participated in the study signed an informed consent form which explained the study objective, the intervention to be performed and the expected results. The confidentiality of the study subjects was also respected by having the data stored in a database which met the privacy criteria set down in the Organic Act 15/1999, of 13 December, on Personal Data Protection. The study was approved by the Research Ethics Committee of the Jaén hospital complex.
Variables:
- •
Anthropometric variables: sex as a dichotomous variable (male or female); age as a continuous variable and as a variable categorized into four other groups: <15 years of age, between 16 and 40 years, between 41 and 65 years, and over 65 years of age.
- •
Laboratory methods: samples of first morning urine were taken and were stored frozen at −20°C until shipment to the reference laboratory, where urinary iodine was measured using the Benotty procedure, which has been validated and adapted by the laboratory of the Instituto de Investigación Biomédica in Málaga.15
- •
Subjects were asked about the type of salt used at their homes, and answers were categorized as iodized salt, kitchen salt, sea salt, or do not know/did not answer. These answers regarding the type of salt used at home were assumed to be accurate.
Statistical analysis: the data were analyzed using SPSS version 15.0 software. A descriptive analysis of urinary iodine levels was made, including minimum, maximum, mean, median, and standard deviation. The association between urinary iodine and sex, age, and type of salt used was analyzed using Pearson's correlation and a linear regression test. A value of p<0.05 was considered statistically significant.
ResultsA total of 1011 subjects were initially recruited. Eight of these subjects were excluded because basic variables could not be collected or because they had recently undergone diagnostic tests with the use of iodinated contrast agents, information which had not been recorded in the patient history and which we were unaware of, so preventing us from excluding them at the start. The sample consisted of 425 males and 578 females with a mean age of 45.64 years (range, 1–94 years). Table 3 shows their age and sex distribution.
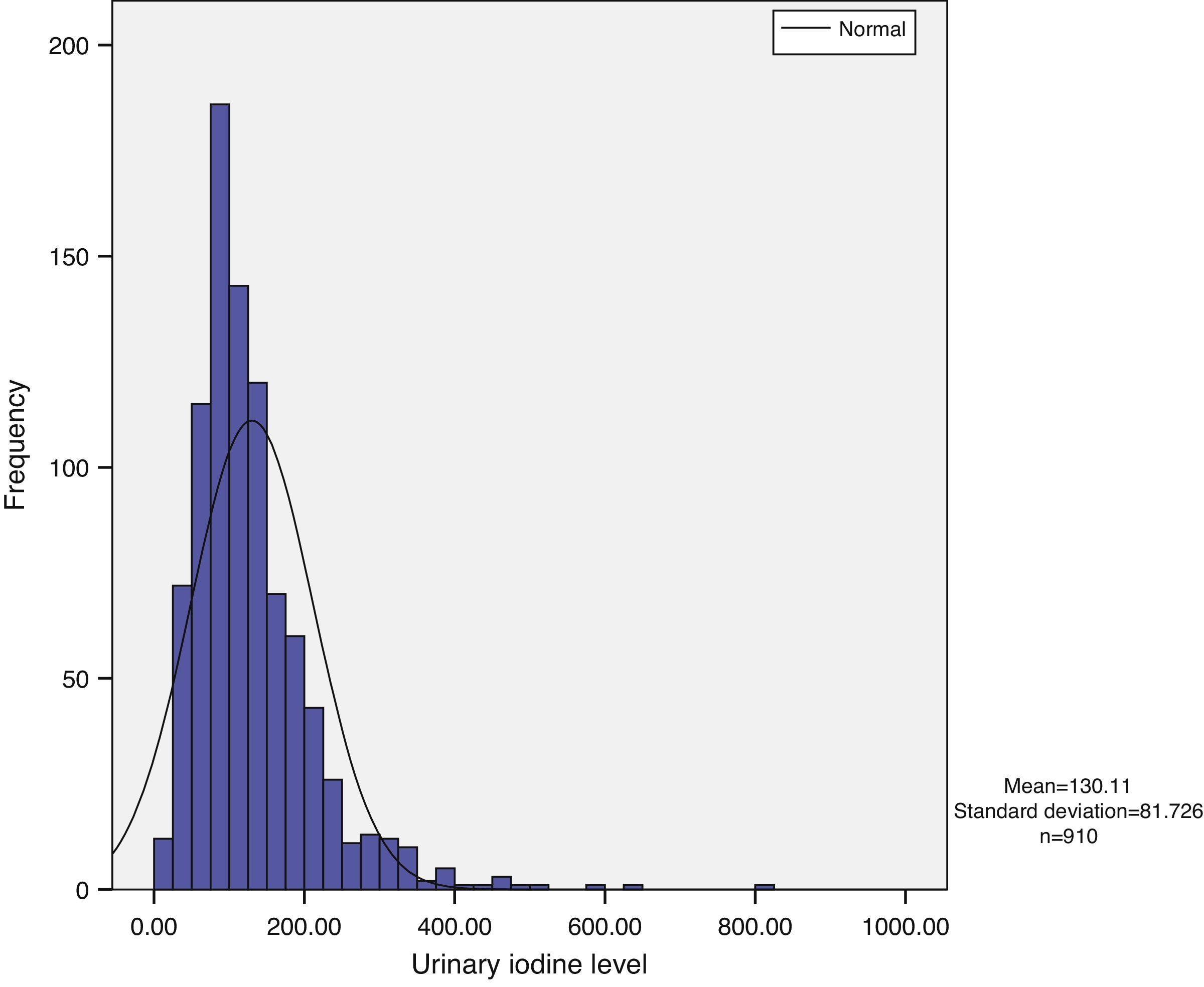
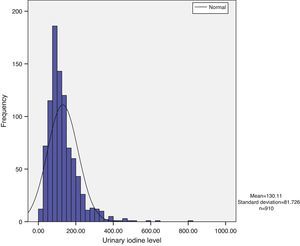
Urinary iodine does not show a normal distribution, but has a long tail to the right, with the tail on the left being narrower. Median urinary iodine in the sample was 110.59μg/L, while the mean level was 130.11μg/L (Fig. 1).
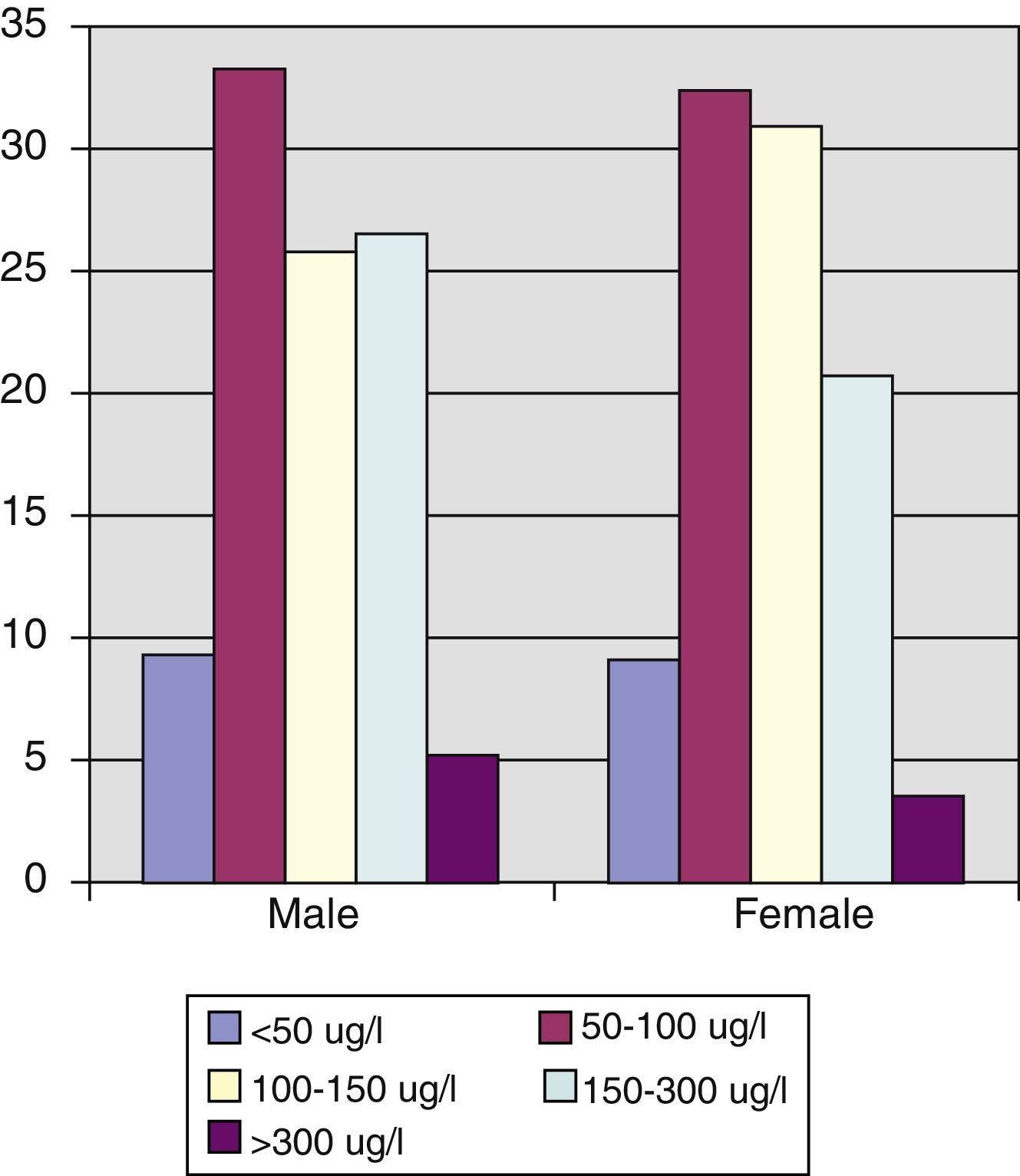
Statistically significant differences in urinary iodine levels were found in schoolchildren as compared to all other age groups. Thus, mean urinary iodine in schoolchildren was 161.52μg/L (CI: 140.98–182.05μg/L), as compared to 145.37 (CI: 134.80–155.93μg/L) in subjects aged 15–40 years, 125.01μg/L (CI: 117.45–132.54μg/L) in those aged 41 to 65 years, and 109.33μg/L (CI: 96.75–121.50μg/L) in those aged >65 years (Spearman's correlation coefficient –0.241, p=0.001) (Fig. 2) (Table 3).
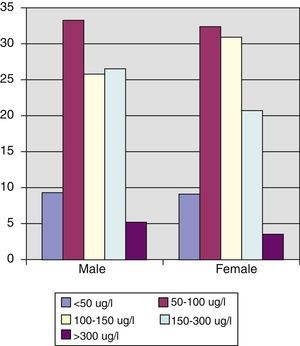
Urinary iodine levels were less than 100μg/L in 43% of the population, and 66.8% of women of childbearing age had levels less than 150μg/L, with median and mean urinary iodine levels of 124.66μg/L and 148.52μg/L respectively (interquartile range, 90.24–177.31μg/L).
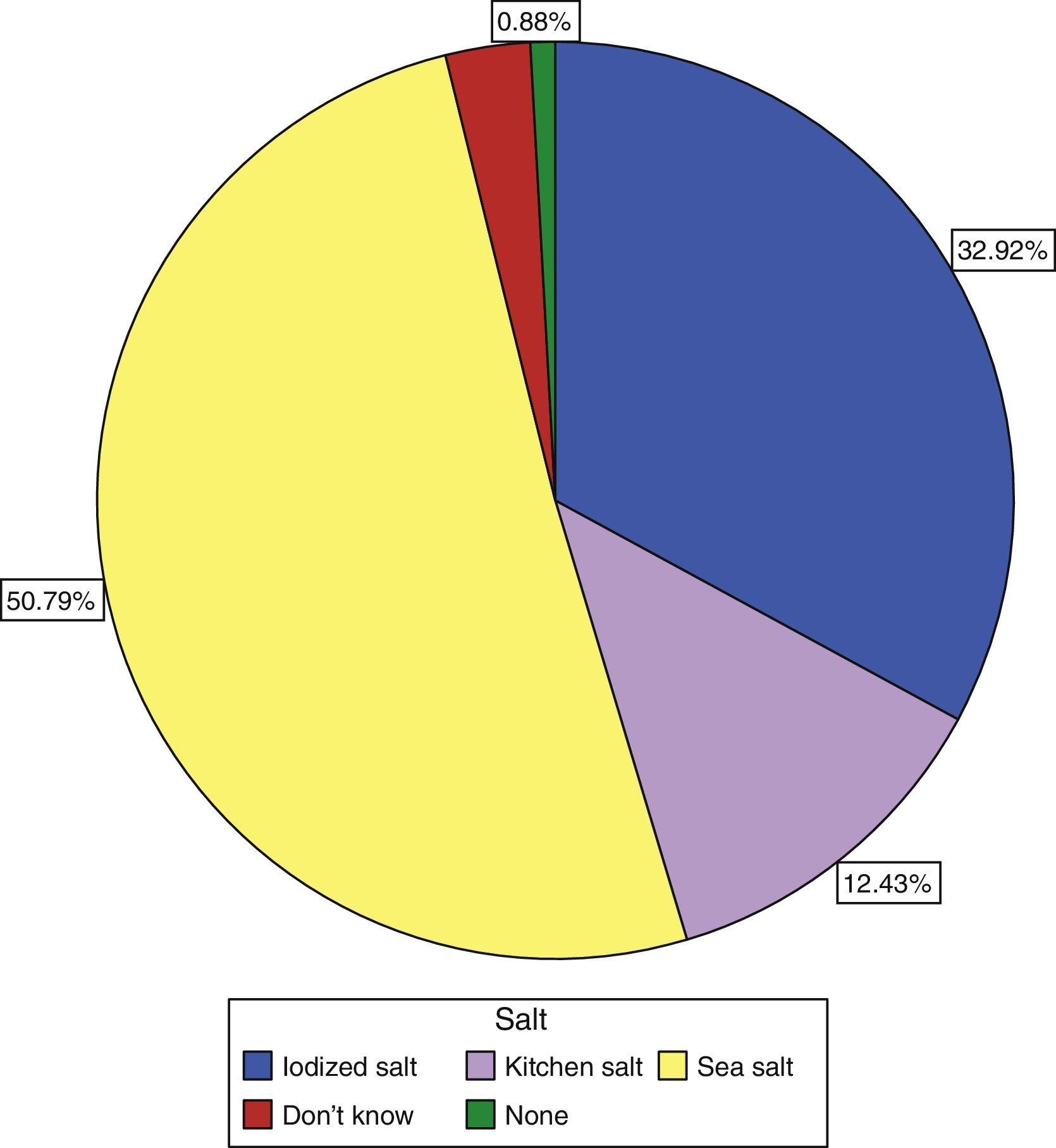
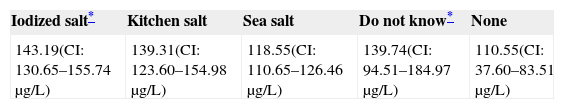
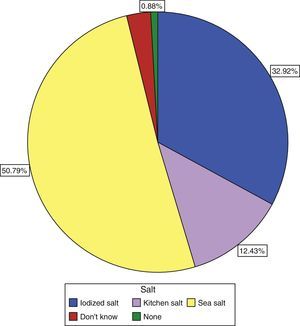
Only 30.9% of subjects surveyed stated that they used iodized salt daily, while 50.8% reported the use of sea salt, and 12.43% of kitchen salt (Fig. 3). Subjects who reported the use of iodized salt had significantly higher urinary iodine levels than all other subjects: 143.19μg/L vs 139.3μg/L in those using kitchen salt, 118.55μg/L in those using sea salt, and 110μg/L (p=0.005) in subjects with no salt consumption, only comparable to the level found in those who did not know the type of salt used: 139.74μg/L; these results are shown in Table 3.
DiscussionThere is a comprehensive literature available in Spanish1–4,14,16 demonstrating the significance of ID in our country. In addition, many studies have related this deficiency to below average intellectual development in children born to mothers living in areas with ID5,17,18 and with hypothyroxinemia19 as the cause of these minor neurological defects.
Thanks to the recommendations of international organizations such as UNICEF, ICCID, and the WHO,12 iodine nutrition status has improved worldwide. Europe is the region where the greatest prevalence of ID is found (52%), and where iodized salt coverage is lowest (approximately 25%). Moreover, many European countries have no programs for controlling ID.20
The working group “Thyroid dysfunction and iodine deficiency” of the Spanish Society of Endocrinology and Nutrition (SEEN) published a monograph addressing ID status in Spain. In the final chapter, devoted to ID prevention in the public health setting, it recommended the adequate use of iodized salt in the general population. It also emphasized the need to inform health care professionals of the importance of maintaining adequate iodine nutrition in pregnant women, and recommended the use of iodine supplements of up to 200μg daily in this population.21
Different studies have been conducted in Jaén on the iodine nutrition status of the population. Thus, in a first study22 conducted in schoolchildren we reported a median urinary iodine level of 90μg/L (mean, 110μg/L) and a prevalence of goiter of approximately 20%, very similar to that reported in prior studies.23 A subsequent study conducted in pregnant women found urinary iodine levels to be much lower than the WHO recommended, with a median of 108.34μg/L and a mean of 128.57μg/L. Levels lower than 100μg/L and than 50μg/L were found in 45.5% and 14% of pregnant women respectively.14 These studies showed that the population reporting the use of iodized salt had significantly higher urinary iodine levels (143.19μg/L), which explains the differences seen in this study between iodized salt users and all other groups. Mean urinary iodine level in schoolchildren has improved over time to 161.52μg/L (median, 149.00μg/L), and is therefore within the optimum range of iodine nutrition according to WHO indicators.
This occurred with no increased consumption of iodized salt, which continues to be low as compared to that of other populations and much lower than that recommended by official bodies (only 30.9%).12 This suggests the existence of a so-called “silent iodine prophylaxis” which has caused a trend to an improvement in iodine nutrition indicators which may be attributable to the consumption of iodine-containing food such as milk and dairy products.24–27 It may also be that, because of the lack of a reliable verification of consumption of a type of salt other than iodized salt, such as sea or kitchen salt, this is the result of a confusion of surveyed subjects when recording the results in the dietary survey.
As to why schoolchildren have significantly higher urinary iodine levels than adults, on the one hand, this is probably related to greater milk ingestion and, on the other hand, to the fact that salt consumption is usually restricted at more advanced ages.
No differences were found in urinary iodine levels between adult men and women, but it should be noted that almost 66% of women of childbearing age had levels less than the 150μg/L recommended by the WHO, and 30% had values lower than 100μg/L, which suggests that their iodine nutrition is deficient and that iodine supplementation during pregnancy and breast-feeding is indicated in these cases. In a prior study14 we found that pregnant women who had been using iodized salt over a long period had median basal urinary iodine levels in the first trimester of pregnancy of 169.78μg/L vs 94.17μg/L (p=0.014), which led us to conclude that the recommendation that the general population use iodized salt could well be sufficient for maintaining adequate urinary iodine levels in pregnant women, without the need for iodine supplements. However, because of the small sample size and the limited nature of this study, this conclusion should be treated with caution, and the recommendation that pregnant women take iodine supplements should be maintained for the time being.
As regards overall urinary iodine levels, our results were significantly lower than those reported by other authors; thus, the study conducted in Catalonia found a mean urinary iodine level of 182.81μg/L,28 and the “Tirobus” study, published in 2010, found an overall mean urinary iodine level of 167.9μg/L.24 Moreover, in the most recent study, in a population from Asturias (pending publication), the median urinary iodine level in schoolchildren was 180.7μg/L, with a mean value greater than 200μg/L, while only 16% of children had levels less than 100μg/L (personal communication, Dr. E. Menéndez).
However, despite the requests made to Andalusian health authorities and to the national Ministry of Health and the commitment of the Interterritorial Council of the National Health System (CISNS) of December 2003 to implement the recommendations issued by the different scientific bodies, the only actions so far taken have been the use of iodized salt in school canteens29 and the recognition of the need for iodine supplementation in pregnant women to prevent disorders caused by ID in their offspring.30 By the same token, no protocols have been implemented for the continued evaluation of iodine nutrition status in our region, and there are no campaigns to encourage the population to apply effective iodine prophylaxis through iodized salt consumption. By contrast, in Asturias8,9 and the Basque Country,31 acceptable urinary iodine levels in schoolchildren and the introduction of iodized salt consumption into almost 90% of homes in Asturias and 53% in the Basque Country have been achieved.
Thus, it should be stated that in Andalusia, any initiative in this regard is always taken by the researchers without official support. It was for this reason, and as a continuation of our research line, that a little over one year ago we decided to go one step further and to analyze not only schoolchildren or pregnant women, who are the priority objective as regards the optimization of iodine nutrition, but also the general population to ascertain iodine nutrition status and the extent of iodized salt consumption. The strength of this study lies in the fact that a population sample highly representative of the whole province of Jaén was enrolled, including subjects of both sexes and different ages. Recruitment bias was minimized because the researchers were the same at all times, and because they did not delegate data collection to staff outside the study.
The main study weakness is that the study subjects had originally required the health care services of the Andalusian Health Service, which may suggest the possibility of abnormal results, given their status as “not 100% healthy”. This was minimized by excluding subjects with diseases or conditions as detailed in “Subjects and methods” section.
The analysis of iodine nutritional status in our region is particularly important if we are to achieve an improvement in ID indicators such as the prevalence of goiter in schoolchildren and the median urinary iodine level. At the same time, to achieve this improvement, health care authorities must first become aware of the magnitude of the problem and epidemiological mechanisms leading to the resolution of ID status need to be implemented, mainly through the promotion of iodized salt consumption. There has been controversy in recent times as to whether or not iodine supplementation is needed in pregnant women, and defects have been found both in the labeling of some salts sold and in their iodine content.32 It is, therefore, important to get health care authorities involved in the implementation of campaigns to solve this problem.
To sum up, it may be stated that the current iodine nutrition status in Jaén can be considered adequate, but that the proportion of the population with urinary iodine levels less than 100μg/L is still very high, and that iodized salt consumption in the home is still much lower than the consumption considered optimal by the WHO.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Olmedo Carrillo P, García Fuentes E, Gutiérrez Alcántara C, Serrano Quero M, Moreno Martínez M, Ureña Fernández T, et al. Evaluación del estado de nutrición yódica en población general en la provincia de Jaén. Endocrinol Nutr. 2015;62:373–379.