Virilizing ovarian tumors are very infrequent, representing less than 0.2% of all cases of hyperandrogenism and less than 1% of ovarian tumors.1 Two women with severe hyperandrogenism who turned out to have a Leydig cell tumor are reported.
Our first patient was a 50-year-old woman with a 5-year history of weight gain of 20kg, secondary amenorrhea, acne, hirsutism and progressive virilization (male-pattern alopecia, clitoromegaly and deepening of the voice). On physical examination, she revealed moon facies, buffalo hump, thin and wrinkled skin and abdominal purple red striae. Ferriman–Gallwey hirsutism score was 18. Plasma ACTH and cortisol, and urinary free cortisol were increased. Hormonal assessment is summarized in Table 1. Very high testosterone, slightly high androstenedione (4.59ng/mL, normal 0.3–3.5) but normal dehydroepiandrosterone sulfate (DHEAS) levels (1.25mcg/mL, normal 0.35–4.3) were detected. FSH and LH levels were 27.4 and 13.4U/L respectively (normal range for postmenopause 20–100IU/L). Dexamethasone suppression tests (1 and 8mg) were diagnostic of Cushing's disease; magnetic resonance imaging (MRI) revealed a 7mm microadenoma in the right lateral margin of the pituitary gland, which was resected by transsphenoidal surgery. One month after surgery, she complained of no improvement of her symptoms. Remarkably high testosterone levels, slightly elevated androstenedione (3.89ng/mL) but normal DHEA-S (2.15mcg/mL) were found (Table 1). These findings could indicate either an ovarian origin of hyperandrogenism or persistence of Cushing's disease. Although other diagnoses, such as ovarian hyperthecosis, could not be excluded, the rapid onset and very high levels of androgens suggested an ovarian tumor. Pituitary MRI could match with either tumor persistence or postsurgical changes; neck, thorax and abdominal CT scan and pelvic transvaginal ultrasound failed to find any mass. Bilateral laparoscopic oophorectomy was performed. A pure Leydig cell tumor of 12mm was found on histological examination of the left ovary. Alpha-inhibin immunohistochemical staining was found to be positive. Total and free testosterone levels returned to the normal range (Table 1), and the patient referred improvement in her voice, hair loss, acne and hirsutism, together with development of hot flushes. As hormonal findings suggested persistent Cushing's disease, MRI was performed showing a focal area of low intensity signal on non-contrast T1 suggesting a remaining microadenoma. Some months later the patient finally agreed to a new transsphenoidal pituitary surgery; unfortunately, the adenoma was not found and hypercortisolism persisted (Table 1). A new MRI revealed a 3mm microadenoma in the posterolateral right area of the pituitary; Gamma Knife radiosurgery was then delivered, with a maximum irradiation dose of 33.33Gy. Along the following months the patient lost 10kg, her general condition improved and hormonal levels finally normalized (Table 1).
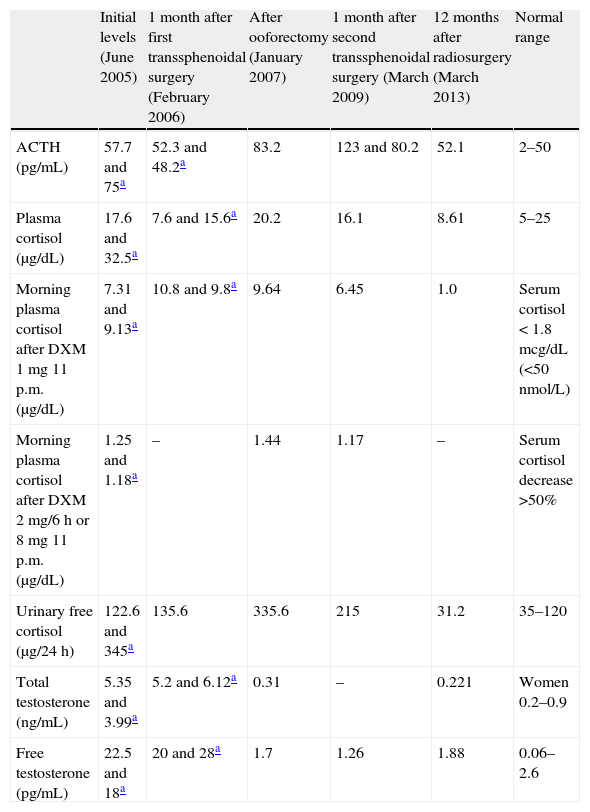
Hormonal levels in Patient 1 along evolution.
| Initial levels (June 2005) | 1 month after first transsphenoidal surgery (February 2006) | After ooforectomy (January 2007) | 1 month after second transsphenoidal surgery (March 2009) | 12 months after radiosurgery (March 2013) | Normal range | |
| ACTH (pg/mL) | 57.7 and 75a | 52.3 and 48.2a | 83.2 | 123 and 80.2 | 52.1 | 2–50 |
| Plasma cortisol (μg/dL) | 17.6 and 32.5a | 7.6 and 15.6a | 20.2 | 16.1 | 8.61 | 5–25 |
| Morning plasma cortisol after DXM 1mg 11 p.m. (μg/dL) | 7.31 and 9.13a | 10.8 and 9.8a | 9.64 | 6.45 | 1.0 | Serum cortisol<1.8mcg/dL (<50nmol/L) |
| Morning plasma cortisol after DXM 2mg/6h or 8mg 11 p.m. (μg/dL) | 1.25 and 1.18a | – | 1.44 | 1.17 | – | Serum cortisol decrease >50% |
| Urinary free cortisol (μg/24h) | 122.6 and 345a | 135.6 | 335.6 | 215 | 31.2 | 35–120 |
| Total testosterone (ng/mL) | 5.35 and 3.99a | 5.2 and 6.12a | 0.31 | – | 0.221 | Women 0.2–0.9 |
| Free testosterone (pg/mL) | 22.5 and 18a | 20 and 28a | 1.7 | 1.26 | 1.88 | 0.06–2.6 |
DXM, dexamethasone.
The second patient was a 60-year-old woman referred to the Endocrinology clinic due to 5-year complaints of male pattern alopecia, hirsutism and deepened voice. Hormonal evaluation showed increased total (8.58 and 4.86ng/mL) and free testosterone levels (16 and 13.7pg/mL); gonadotropin levels were low for postmenopausal state (luteinizing hormone 2.5IU/l, follicle-stimulating hormone 3.84IU/l). Other androgens remained within the normal range: androstenedione 2.92ng/mL, DHEAS 0.81mcg/mL. A CT scan and transvaginal ultrasound revealed no enlargement or mass in abdomen or pelvis. Considering the differential diagnosis of ovarian hyperthecosis vs ovarian tumor both ovaries were removed. A Leydig cell tumor of 15mm was found in the right ovary, with positive staining for alpha-inhibin. After surgery, free and total testosterone levels fell to 0.7pg/mL and 0.22ng/mL. The physical changes gradually reversed and she suffered hot flushes.
Virilizing ovarian tumors are an unusual cause of hyperandrogenism; however, rapidly worsening signs of virilization in a postmenopausal woman should prompt an urgent diagnostic work-up for an androgen-secreting tumor. Peripheral total testosterone higher than 2ng/mL (>7nmol/l), or 3–4 times higher than the upper limit of normal range may be a cut-off level for ovarian neoplasm suspicion.2 One study has shown that testosterone level >8.67nmol/l (2.5ng/mL) had 100% sensitivity for ovarian neoplasm, together with 98% specificity.3 Our initial differential diagnosis also considered stromal hyperthecosis. Pure Leydig cell tumors typically occur in postmenopausal women, with hirsutism or virilization in 75% of cases. These tumors are typically small at presentation,1 so imaging studies are often not useful for the diagnosis,6 as happened in our cases. Less than 150 cases have been reported to date.4,5
In our first case, differential diagnosis of hyperandrogenism was particularly challenging, as our patient also suffered from Cushing's disease with an awkward evolution. However, as adrenal glands produce up to 95% of DHEA-S, its normal levels ruled out an adrenal origin of hyperandrogenism, while high testosterone levels indicated a virilizing ovarian tumor. Coexistent adrenal and ovarian hyperandrogenism has been reported in other unusual settings.7 Rare steroid cell ovarian tumors of adrenocortical type have also been reported to result in cortisol co-secretion.8 Ovarian tumors may also be associated with Cushing's syndrome due to ectopic secretion of ACTH or corticotropin-releasing factor (CRF).9,10 Our patient showed an ACTH-dependent Cushing's syndrome, which ruled out the possibility of cortisol co-secretion. Besides, hyperandrogenism disappeared after tumor removal, while Cushing's disease persisted; therefore, an ectopic source of ACTH secretion could also be ruled out.
In conclusion, screening for virilizing ovarian tumors is mandatory in rapidly progressive virilization, with elevated testosterone levels, after ruling out other more frequent causes. In the unusual situation of coexisting hypercortisolism, DHEA-S can be useful for differential diagnosis, as normal levels are found when the androgen source is ovarian. Normal imaging does not rule out virilizing ovarian tumors.
Please cite this article as: Ballesteros-Pomar MD, Vidal-Casariego A. Una causa oculta de virilización en mujeres postmenopaúsicas. Endocrinol Nutr. 2014;61:436–438.