Secondary osteoporosis encompasses a wide group of heterogeneous disorders. Secondary osteoporosis may pose diagnostic problems that need to be addressed in order to establish adequate treatment and prognosis. The causes of secondary osteoporosis include endocrine, hematological, and conjunctive tissue disorders, drug treatments, kidney diseases, and nutritional and gastrointestinal disorders, amongst others.1 Approximately 40–60% of cases of osteoporosis in males are secondary, and their most common causes include glucocorticoid treatment, hypogonadism, and alcoholism.2
In elderly subjects, multiple myeloma (MM) and other hematological tumors induce a clinical picture similar to primary osteoporosis. This condition should therefore be ruled out in patients with fragility fractures and a fast clinical course.3 In MM, osteoclastic activity is increased because myeloma cells cause or induce the production of osteoclastogenic factors in a bone microenvironment and decrease the production of osteoprotegerin (OPG), a decoy receptor of RANK ligand (RANKL), by osteoblastic cells.4 RANKL increase enhances the formation and survival of osteoclasts and macrophage inflammatory protein-1 (MIP-1α) acts as a chemotactic factor for osteoclast precursors, and also promotes the growth and survival of MM cells. On the other hand, the bone destruction process releases growth factors that increase MM cell proliferation, thus exacerbating the osteolytic process.5 In addition to the role of RANKL in bone resorption increase, bone formation inhibition occurs in myeloma. TGF-beta, as well as inhibitors of the relevant wnt pathway such as DKK-1, sFRP-2, and sFRP-3, produced by myeloma cells, have recently been implicated.6
In most patients with MM, skeletal manifestations (osteopenia, fractures, and osteolysis) are common and lead to an impaired quality of life. Bone lesions in myeloma differ from other lytic metastatic lesions in their suppression or absence of osteoblastic activity in an area with a high tumor burden. These lesions are best visualized using MRI or conventional X-rays.7 Other techniques such as 99mTc scintigraphy are not more helpful as compared to conventional X-rays because they underestimate bone lesions in patients with multiple myeloma.
Case reportWe report the case of an 82-year-old male patient referred to the bone metabolism clinic for work-up for osteoporosis because he had vertebral fractures at T12 and L1, L2, and L4. His personal history included hyperuricemia, hypertension, and benign prostatic hyperplasia monitored by urology. The patient reported that his mother had died at a young age from a tumor he could not specify, and that three of his siblings had died from hepatocarcinoma.
The patient reported severe lumbar pain for approximately six months, which had increased in severity and become disabling. He did not report prior trauma, was a former smoker and a former alcohol drinker, and had not previously received corticoid treatment, except in the last month for lumbar pain. The patient had experienced a weight loss of 8kg in one year, associated with hyporexia. A dietary survey revealed a low intake of dairy products, approximately one serving daily, and low sun exposure. Physical examination showed tenderness in the lumbar spine and costal area, and pain with movement. Weight was 52.5kg and height 1.54m (with moderate kyphosis), with a body mass index of 22.13kg/m2. Routine blood chemistry showed kidney failure, with creatinine 1.7mg/dL and urea 72.8mg/dL (previously unknown), hypercalcemia (calcium level of 10.8mg/dL), phosphate levels of 3.9mg/dL, and alkaline phosphatase levels of 46U/L.
A secondary cause of osteoporosis was suspected because of hypercalcemia and the patient's signs and symptoms, while the protein electrophoresis requested showed hypoproteinemia (6g/dL), albumin within the normal range (4.4g/dL; NV, 3.5–5g/dL) and alpha-1 and alpha-2 globulin increases of 5% (NV, 2.9–4.5) and 15.4% (NV, 7.1–11.8%) respectively. Beta-globulin was normal 10.8% (NV, 7.9–13.7%) and gamma peak was below the normal range 7% (NV, 11.1–18.8). Serum light chain levels were 48,200mg/dL (NV, 0.33–1.94), with low levels of immunoglobulins IgA, M, and G, supporting the diagnosis of Bence Jones proteinuria in the setting of light chain myeloma.
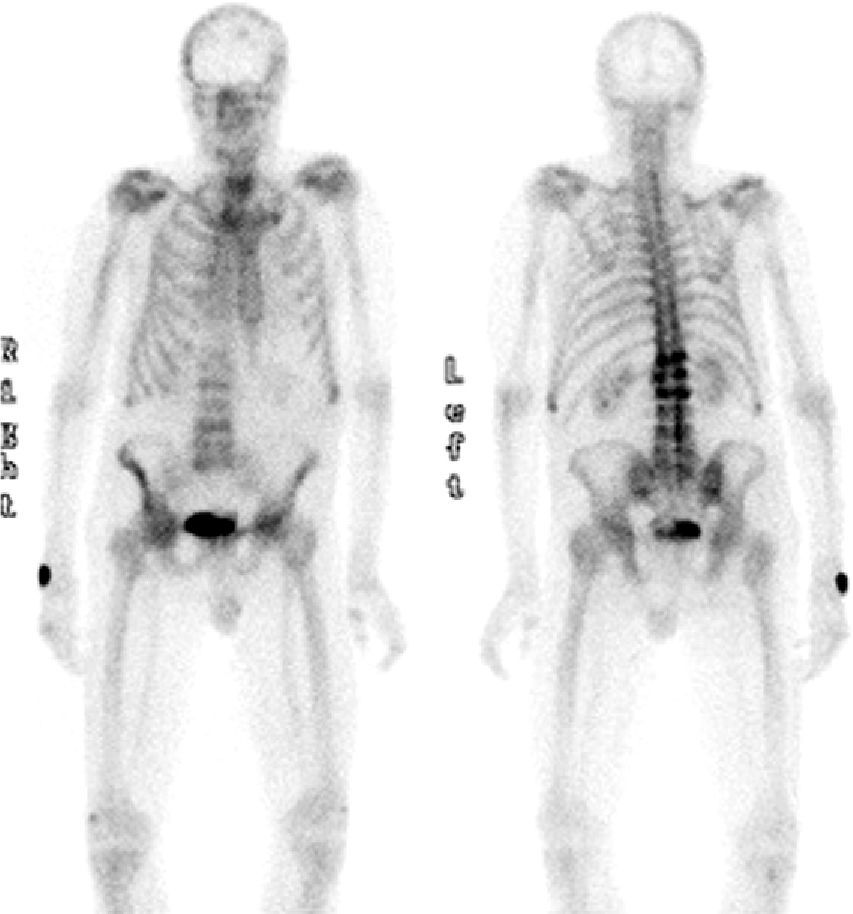
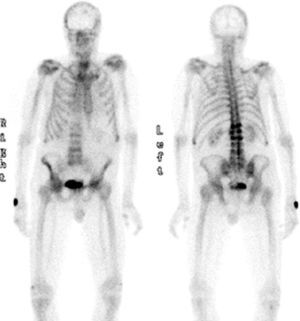
The PSA level was 4ng/mL, and the TSH level 1.56mIU/mL. A bone metabolism study revealed low vitamin D levels (25OHD D, 8.2ng/mL) with an iPTH level of 21pg/mL (NV, 15–65pg/mL). A complete blood count showed normocytic normochromic anemia, as well as leukopenia (3.870 WBCs). The erythrocyte sedimentation rate was 27mm. X-rays of the thoracic spine and the lumbar spine showed multiple vertebral fractures at T12 (grade 3), L1, L2, and L4 (grade 2). Bone densitometry showed a lumbar T score of −1.9 SD, −2.7 SD in total hip, and −0.6 SD in femoral neck, and bone scintigraphy revealed high uptake in T12, L1, L2, and L4 (Fig. 1).
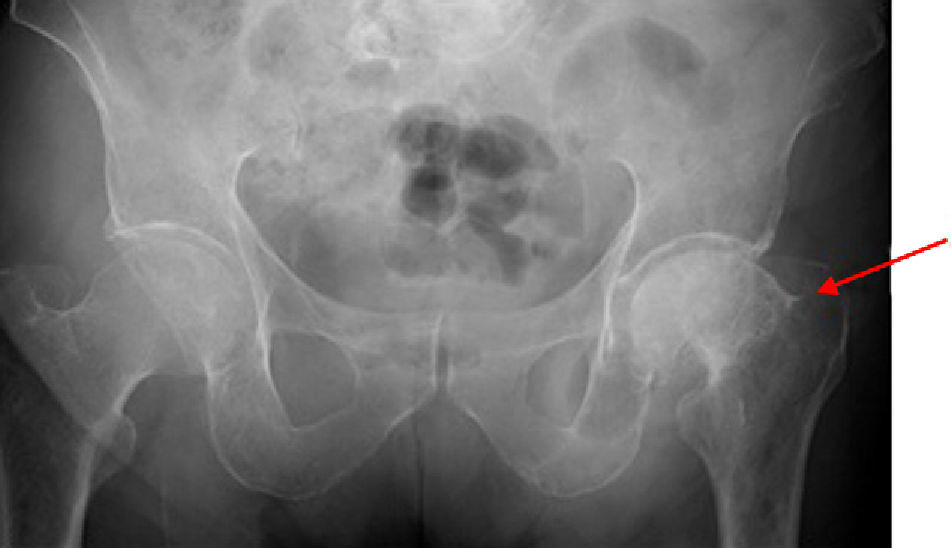
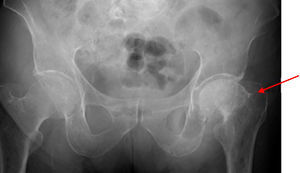
During the study, the patient spontaneously reported pain and functional impotence in the left lower limb. Radiographic study was therefore completed, showing a previously unknown hip fracture and multiple lytic lesions in both femoral shafts (Fig. 2). The patient's course was complicated by respiratory tract infection and severe respiratory failure that did not respond to treatment and eventually caused death. Bone marrow puncture could not be performed.
DiscussionTwenty to thirty percent of osteoporotic fractures occur in males, and it is estimated that 13–25% of males over 50 years of age will experience an osteoporotic fracture during their lives.8 The need to rule out secondary osteoporosis is even greater in males because in approximately 50% of cases osteoporosis is secondary to other conditions, including alcoholism, rheumatic disease, immobilization, glucocorticoid treatment, endocrine disease, and hematological neoplasms such as MM. MM is characterized by monoclonal neoplastic proliferation of the plasma cells leading to bone destruction with osteolytic lesions, osteopenia, and pathological fractures. Diagnosis is suspected based on bone pain with radiographically evident lytic lesions, increased serum protein levels or the presence of monoclonal protein in urine or plasma, systemic signs or symptoms of malignancy such as unexplained anemia, and kidney failure. Most patients have symptoms or signs related to plasma cell infiltration in bone and other organs, and kidney damage from excess light chains. Our patient had all of these signs.
Excess bone destruction is the most common cause of morbidity in patients with MM. Recent studies have shown new biological mechanisms accounting for bone involvement in these patients. Thus, heparinase expression by myeloma tumor cells enhances local bone destruction, and also increases systemic osteoclastogenesis and osteolysis mimicking systemic osteoporosis.9 A positive correlation has been shown between the heparinase expression level and RANKL levels. In addition, other studies have shown the presence of “hybrid cells” in patients with myeloma by the detection in biopsies of fragments of nuclei from tumor cells in osteoclasts.10 These hybrid cells may lead to the “reprogramming” of osteoclasts and contribute to an increased bone resorption as compared to normal osteoclasts.
As regards survival, some studies show a relationship with bone mineral density, as measured by lumbar spine quantitative computed tomography.11 Thus, patients with a T-score≤3.5 SD die approximately 18 months earlier than those with T-scores≥3.5 SD.
Myeloma patients have an increased risk of infection due to immune system dysfunction caused by impaired lymphocyte function, hypogammaglobulinemia, and the suppression of normal plasma cell function. In fact, respiratory tract infection was the cause of death in our patient, who had an uncommon kappa light chain variant with Bence Jones proteinuria. As regards treatment, radiotherapy is reserved for the control of local pain but is not curative. Percutaneous vertebroplasty is used to stabilize lesions and relieve pain. Kyphoplasty reduces deformity and stabilizes vertebral fracture. Bisphosphonates are the most commonly used medical treatment. Intravenous zoledronate regimens have now shown a greater efficacy for controlling bone disease in these patients.12 New antiosteoporotic agents such as monoclonal antibodies to RANKL represent a promising option for the future.
In conclusion, males with established osteoporosis should undergo careful screening for secondary osteoporosis in order to establish an adequate diagnosis, treatment, and prognosis. A rapidly progressing clinical course is highly suggestive of a hematological neoplasm, such as multiple myeloma, for which specific drug intervention should not be delayed.
Please cite this article as: Suleiman Martos Y, et al. Mieloma múltiple como causa de osteoporosis secundaria con evolución rápidamente progresiva. Endocrinol Nutr. 2012;59:398–400.