Neuroendocrine tumors are a group of neoplasms arising from the neural crest and endoderm and very heterogeneous as regards localization, clinical behavior, aggressiveness, and prognosis. Pancreas and gastrointestinal tract are the most common sites where neuroendocrine tumors can be found.
Material and methodsA review was made of all cases of neuroendocrine tumors diagnosed at Hospital Universitario Clínico San Carlos (HUCSC) from January 2007 to May 2012. Data were compared to the results provided by the Registry of the Spanish Group on Neuroendocrine Tumors (RGETNE).
ResultsThe study cohort comprised 78 patients. Gastroenteric nonfunctional tumors were the most common neoplasms. Metastases were found at diagnosis in 50.6% of patients, with nodal involvement being most prevalent. Tumors located in the rectum were associated to the highestrate of metastasis. Overall 2-year survival rate was 74.8% and was related to sex, Ki-67 expression, and presence of metastasis.
Los tumores neuroendocrinos (TNE) representan un grupo de neoplasias originadas por células de la cresta neural y del endodermo con gran heterogenicidad en cuanto a localización, comportamiento clínico, agresividad y pronóstico. El páncreas y el tubo digestivo constituyen las localizaciones más frecuentes.
Material y métodosSe ha realizado una revisión de casos diagnosticados de neoplasia neuroendocrina gastroenteropancreática (TNEGEP), tanto primaria como metastásica, en el Hospital Universitario Clínico San Carlos (HUCSC) entre enero de 2007 y mayo de 2012. Se han comparado los datos obtenidos con los aportados por el Registro del Grupo Español de Tumores Neuroendocrinos (RGETNE).
ResultadosEl estudio constó de 78 pacientes. El tipo de tumor más común fue el gastroentérico no funcionante. Un 50,6% de los pacientes presentó metástasis al diagnóstico, siendo lo más prevalente la afectación ganglionar. Los TNEGEP localizados en el recto se acompañaron de un mayor porcentaje de metástasis. La supervivencia global a los 24 meses fue del 74,8%, estando en relación con el sexo, la expresión del Ki-67 y la presencia de enfermedad a distancia.
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms derived from neuroendocrine cells with a variable behavior. NETs most commonly occur in the gastrointestinal tract and pancreas.1
They are uncommon neoplasms (2–5 cases per 100,000 inhabitants/year). Their incidence is similar in both sexes. NETs may occur sporadically or as part of a hereditary syndrome (multiple endocrine neoplasia type 1 (MEN-1), Von Hippel-Lindau, neurofibromatosis type 1 or tuberous sclerosis).1
The most recent WHO classification (2010) establishes three grades based on the Ki-67 index: grade 1 (Ki-67≤2%), grade 2 (Ki-67 3%–20%), and grade 3 (Ki-67>20%).2 Metastases are more frequent in grade 3, particularly in liver, bone, and lymph nodes.3
Clinical signs depend on three factors: location, the presence of metastases, and secretory activity.4 Symptom occurrence depends on the specific type of tumor and, in carcinoid syndrome, on the existence of liver metastases.5
NET management depends on the type of tumor and patient characteristics. In localized disease, the treatment of choice is surgery, which is the only curative alternative. Total resection of the lesion with negative margins is a determinant predictor of survival in these tumors.1
In distant disease, surgery of primary tumor, if resectable, is indicated because it improves obstructive and hypersecretory clinical signs.6 Surgery of liver metastases may be curative if they are resectable, or debulking as adjuvant treatment.7
Medical treatments include somatostatin analogs (SAs), interferon, chemotherapy, tyrosine kinase inhibitors (TKIs), and inhibitors of the mammalian target of rapamycin (mTOR).8
The wide diversity of these tumors warrants the need for conducting comparative studies which will make it possible to homogenize their diagnostic and therapeutic management. This review represents a key starting point with its description of a specific NET unit and, despite the small sample size, should facilitate the conduct of subsequent studies. This unit consists of a multidisciplinary team including endocrinologists, oncologists, surgeons, radiologists, nuclear medicine physicians, and pathologists who, in addition to caring for the reference population of the hospital, see patients from other areas of Madrid and all other Spanish regions.
ObjectivesThe primary study objective was to perform a descriptive analysis of cases of gastroenteropancreatic NETs (GEP-NETs) diagnosed at Hospital Universitario Clínico San Carlos (HUCSC) from January 2007 to May 2012 including epidemiological data, presentation, diagnostic process, treatment, and results. As a secondary objective, the discussion will try and assess if the data collected are superimposable on those from the Registry of the Spanish Group on Neuroendocrine Tumors (RGETNE) in an attempt to identify potential prognostic markers of survival.
Patients and methodsSelection of casesTo select patients, a list of all biopsies performed at the hospital from January 2007 to May 2010 diagnosed as tumors of neuroendocrine cells of gastropancreatic origin was requested from the pathology department of HUCSC. The selected clinical histories, provided by the filing, coding, and clinical documentation department of HUCSC, were reviewed.
Inclusion criteriaAll patients diagnosed with both primary and metastatic gastroenteropancreatic neuroendocrine tumors.
Exclusion criteria- -
Patients diagnosed with non-gastroenteropancreatic neuroendocrine tumors.
- -
Patients diagnosed with neuroendocrine cell hyperplasia.
One hundred and nine biopsies from 90 patients were initially selected, but 12 patients were subsequently excluded from the sample, three who had lung NETs, three with neuroendocrine cell hyperplasia, four with lipomas, and two because their clinical history could not be accessed. The final study sample therefore consisted of 78 patients.
Study variablesStudy variables have been reported in relation to four groups of neuroendocrine tumors, specified as such in the pathological study, according to their location and predominant clinical form of presentation:
- -
Incidental,
- -
Gastrointestinal symptoms: abdominal pain, diarrhea, constipation, gastrointestinal bleeding (both upper and lower).
- -
Constitutional symptoms: an association of asthenia, anorexia, and 5% or higher weight loss in 6–12 months. The presence of at least one of the symptoms was considered to represent constitutional symptoms.
- -
Clinical signs: hypoglycemia/hypokalemia: based on laboratory tests performed in the study period, and always before treatment: (a) acid hypersecretion: reported as epigastric pain associated with intake, or attenuated in severity by PPI use; (b) diarrhea; (c) necrolytic migratory erythema; (d) diabetes mellitus: considered regardless of whether or not it could be related to the presence of tumor; (e) carcinoid syndrome: an association of flushing and diarrhea; and (f) carcinoid heart disease: confirmed by echocardiography.
The four categories are thus defined as follows:
- -
Non-functioning gastroenteric neuroendocrine tumor (NFGENET): extrapancreatic gastrointestinal location, with no secretory symptoms.
- -
Functioning gastroenteric neuroendocrine tumor (FGENET): extrapancreatic gastrointestinal location, with secretory symptoms.
- -
Non-functioning pancreatic neuroendocrine tumor (NFPNET): pancreatic location, no secretory clinical signs.
- -
Functioning pancreatic neuroendocrine tumor (FPNET): pancreatic location, secretory clinical signs.
Survival was also studied as a function of disease stage:
- -
Local: no metastatic involvement.
- -
Regional: regional lymph node involvement.
- -
Distant: disseminated metastases.
Qualitative variables are given with the frequency distribution. Quantitative variables are summarized using the mean and standard deviation, or the median and interquartile range (IQR) for variables not following a normal distribution.
Survival functions were estimated using the Kaplan–Meier method. The independent variables selected to study mortality events included tumor type, WHO grade, and disease stage, because they are the most widely studied in the literature. Survival functions in the different subgroups were compared using a Breslow exact test. In all hypothesis tests, the null hypothesis was rejected with a type I error or an α error less than 0.05. SPSS software for Windows version 15.0 was used for statistical analysis.
ResultsThe study sample consisted of 78 patients, 46 men (59%) and 32 women (41%). Mean age at diagnosis was 55.5 years (IQR, 15–86 years). A familial history of interest was collected in 55 patients, of whom 7.5% had a history of MEN-1, 20% of colorectal cancer, and 34.5% of tumors in locations other than the gastrointestinal tract and unrelated to MEN-1 syndrome. The most common personal history in the sample was the presence of other types of tumors (the same definition as for familial history) in 20.8% of patients. In addition, five patients had colorectal cancer and four had MEN-1, the latter being equally distributed between the sexes. Smoking and alcohol consumption data were recorded in 73 patients, of whom 50.7% had smoked at some time before diagnosis and 22.1% had drunk alcohol. Gastroenterology was the main department of origin (32.1%).
The predominant type of tumor was NFGENET (55.8%), which was most common in both sexes. The second most common group consisted of FPNETs (26%). The most common location was the pancreas (35%), but tumors located in the gastrointestinal tract were overall more common (64.9%), predominantly in the rectum (19.5%).
Ki-67 expression status was assessed in 92.3% of patients. Of these, 46.5% were classified as WHO grade 1. This group also had fewer metastases, while distant disease was more common in grade 3. Regional disease and distant disease were seen in 13% and 37.7% of patients respectively, with metastases most commonly occurring in lymph nodes.
Gastrointestinal symptoms were the initial manifestation in 48.7% of patients, while 38.5% remained symptom-free until diagnosis. The median period which elapsed from symptom start to the date of diagnosis was 3 months (IQR, 0–180 months).
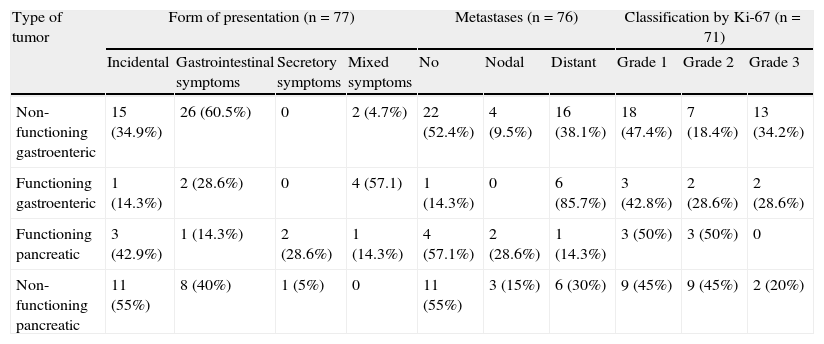
Abdominal pain was the most common symptom, occurring in 39 patients (50%). Constitutional symptoms occurred in 15.4% of tumors. Hypoglycemia was more common in FPNETs, occurring in 42.9%, while diabetes mellitus was found in six FPMETs (30%) and in six NFGENETs (14%), although as noted above, it was not stated whether this was a finding made at diagnosis or at a later time. Symptoms derived from acid hypersecretion and gastrointestinal bleeding were mainly found in patients with NFGENETs. Only one of the FGENETs (14.3%) had no carcinoid syndrome at diagnosis, although all seven patients (100%) had diarrhea and two (28.6%) carcinoid heart disease. No patient was found to have necrolytic erythema (Table 1).
Characteristics by tumor type.
| Type of tumor | Form of presentation (n=77) | Metastases (n=76) | Classification by Ki-67 (n=71) | |||||||
| Incidental | Gastrointestinal symptoms | Secretory symptoms | Mixed symptoms | No | Nodal | Distant | Grade 1 | Grade 2 | Grade 3 | |
| Non-functioning gastroenteric | 15 (34.9%) | 26 (60.5%) | 0 | 2 (4.7%) | 22 (52.4%) | 4 (9.5%) | 16 (38.1%) | 18 (47.4%) | 7 (18.4%) | 13 (34.2%) |
| Functioning gastroenteric | 1 (14.3%) | 2 (28.6%) | 0 | 4 (57.1) | 1 (14.3%) | 0 | 6 (85.7%) | 3 (42.8%) | 2 (28.6%) | 2 (28.6%) |
| Functioning pancreatic | 3 (42.9%) | 1 (14.3%) | 2 (28.6%) | 1 (14.3%) | 4 (57.1%) | 2 (28.6%) | 1 (14.3%) | 3 (50%) | 3 (50%) | 0 |
| Non-functioning pancreatic | 11 (55%) | 8 (40%) | 1 (5%) | 0 | 11 (55%) | 3 (15%) | 6 (30%) | 9 (45%) | 9 (45%) | 2 (20%) |
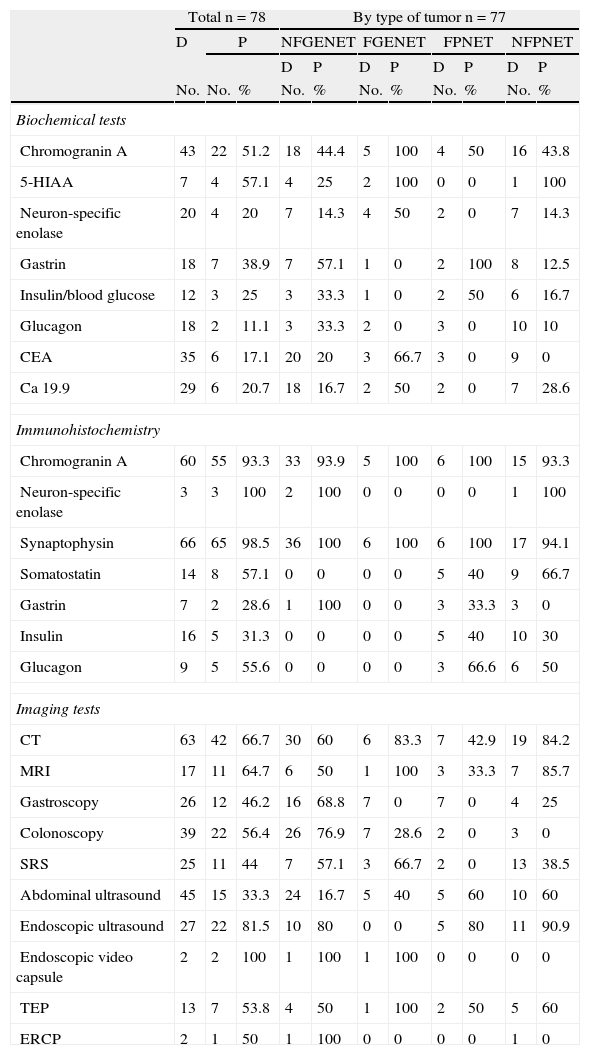
Computed tomography (CT), abdominal ultrasound, and colonoscopy were the tests most commonly performed, and lesions were located in 66.7%, 33.3%, and 56.4% of cases, respectively. Endoscopic ultrasound achieved the greatest location rate, showing the lesion in 87.5% of pancreatic NETs and in 80% of NFGENETs. Gastroscopy and endoscopic video capsule mainly detected gastroenteric tumors. Positron emission tomography (PET) and somatostatin receptor scintigraphy (SRS) were mainly performed in patients with NFPNETs (Table 2).
Diagnostic procedures.
| Total n=78 | By type of tumor n=77 | ||||||||||
| D | P | NFGENET | FGENET | FPNET | NFPNET | ||||||
| D | P | D | P | D | P | D | P | ||||
| No. | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Biochemical tests | |||||||||||
| Chromogranin A | 43 | 22 | 51.2 | 18 | 44.4 | 5 | 100 | 4 | 50 | 16 | 43.8 |
| 5-HIAA | 7 | 4 | 57.1 | 4 | 25 | 2 | 100 | 0 | 0 | 1 | 100 |
| Neuron-specific enolase | 20 | 4 | 20 | 7 | 14.3 | 4 | 50 | 2 | 0 | 7 | 14.3 |
| Gastrin | 18 | 7 | 38.9 | 7 | 57.1 | 1 | 0 | 2 | 100 | 8 | 12.5 |
| Insulin/blood glucose | 12 | 3 | 25 | 3 | 33.3 | 1 | 0 | 2 | 50 | 6 | 16.7 |
| Glucagon | 18 | 2 | 11.1 | 3 | 33.3 | 2 | 0 | 3 | 0 | 10 | 10 |
| CEA | 35 | 6 | 17.1 | 20 | 20 | 3 | 66.7 | 3 | 0 | 9 | 0 |
| Ca 19.9 | 29 | 6 | 20.7 | 18 | 16.7 | 2 | 50 | 2 | 0 | 7 | 28.6 |
| Immunohistochemistry | |||||||||||
| Chromogranin A | 60 | 55 | 93.3 | 33 | 93.9 | 5 | 100 | 6 | 100 | 15 | 93.3 |
| Neuron-specific enolase | 3 | 3 | 100 | 2 | 100 | 0 | 0 | 0 | 0 | 1 | 100 |
| Synaptophysin | 66 | 65 | 98.5 | 36 | 100 | 6 | 100 | 6 | 100 | 17 | 94.1 |
| Somatostatin | 14 | 8 | 57.1 | 0 | 0 | 0 | 0 | 5 | 40 | 9 | 66.7 |
| Gastrin | 7 | 2 | 28.6 | 1 | 100 | 0 | 0 | 3 | 33.3 | 3 | 0 |
| Insulin | 16 | 5 | 31.3 | 0 | 0 | 0 | 0 | 5 | 40 | 10 | 30 |
| Glucagon | 9 | 5 | 55.6 | 0 | 0 | 0 | 0 | 3 | 66.6 | 6 | 50 |
| Imaging tests | |||||||||||
| CT | 63 | 42 | 66.7 | 30 | 60 | 6 | 83.3 | 7 | 42.9 | 19 | 84.2 |
| MRI | 17 | 11 | 64.7 | 6 | 50 | 1 | 100 | 3 | 33.3 | 7 | 85.7 |
| Gastroscopy | 26 | 12 | 46.2 | 16 | 68.8 | 7 | 0 | 7 | 0 | 4 | 25 |
| Colonoscopy | 39 | 22 | 56.4 | 26 | 76.9 | 7 | 28.6 | 2 | 0 | 3 | 0 |
| SRS | 25 | 11 | 44 | 7 | 57.1 | 3 | 66.7 | 2 | 0 | 13 | 38.5 |
| Abdominal ultrasound | 45 | 15 | 33.3 | 24 | 16.7 | 5 | 40 | 5 | 60 | 10 | 60 |
| Endoscopic ultrasound | 27 | 22 | 81.5 | 10 | 80 | 0 | 0 | 5 | 80 | 11 | 90.9 |
| Endoscopic video capsule | 2 | 2 | 100 | 1 | 100 | 1 | 100 | 0 | 0 | 0 | 0 |
| TEP | 13 | 7 | 53.8 | 4 | 50 | 1 | 100 | 2 | 50 | 5 | 60 |
| ERCP | 2 | 1 | 50 | 1 | 100 | 0 | 0 | 0 | 0 | 1 | 0 |
P: positive or elevated; D: done; FGENETs: functioning gastroenteric tumors; NFGENETs: non-functioning gastroenteric tumors; FPNETs: functioning pancreatic tumors; NFPNETs: non-functioning pancreatic tumors.
As regards biochemical tests, serum chromogranin A (CgA) levels were tested in 43 patients, and were increased in 51.2%. 5-Hydroxyindoleacetic acid was tested in a low proportion of patients, most of them with gastroenteric tumors. It should be noted that CgA and 5-HIAA levels were increased in 100% of the FGENETs in which they were tested. Neuron-specific enolase (NSE) was measured in 20 patients, and exceeded the reference levels in 20% of them. Gastrin was tested in 18 patients, and high levels were found in the only gastrinoma studied and in some other types of tumors (Table 2).
As regards immunohistochemistry, CgA was positive in 100% of functioning NETs and in 93.6% of nonfunctioning NETs. NSE was measured in 3.8% of the samples and was positive in all of them. Synaptophysin was negative in only one of the 66 patients tested. Specific markers (somatostatin, insulin, gastrin, and glucagon) were expressed in their corresponding FPNETs (Table 2).
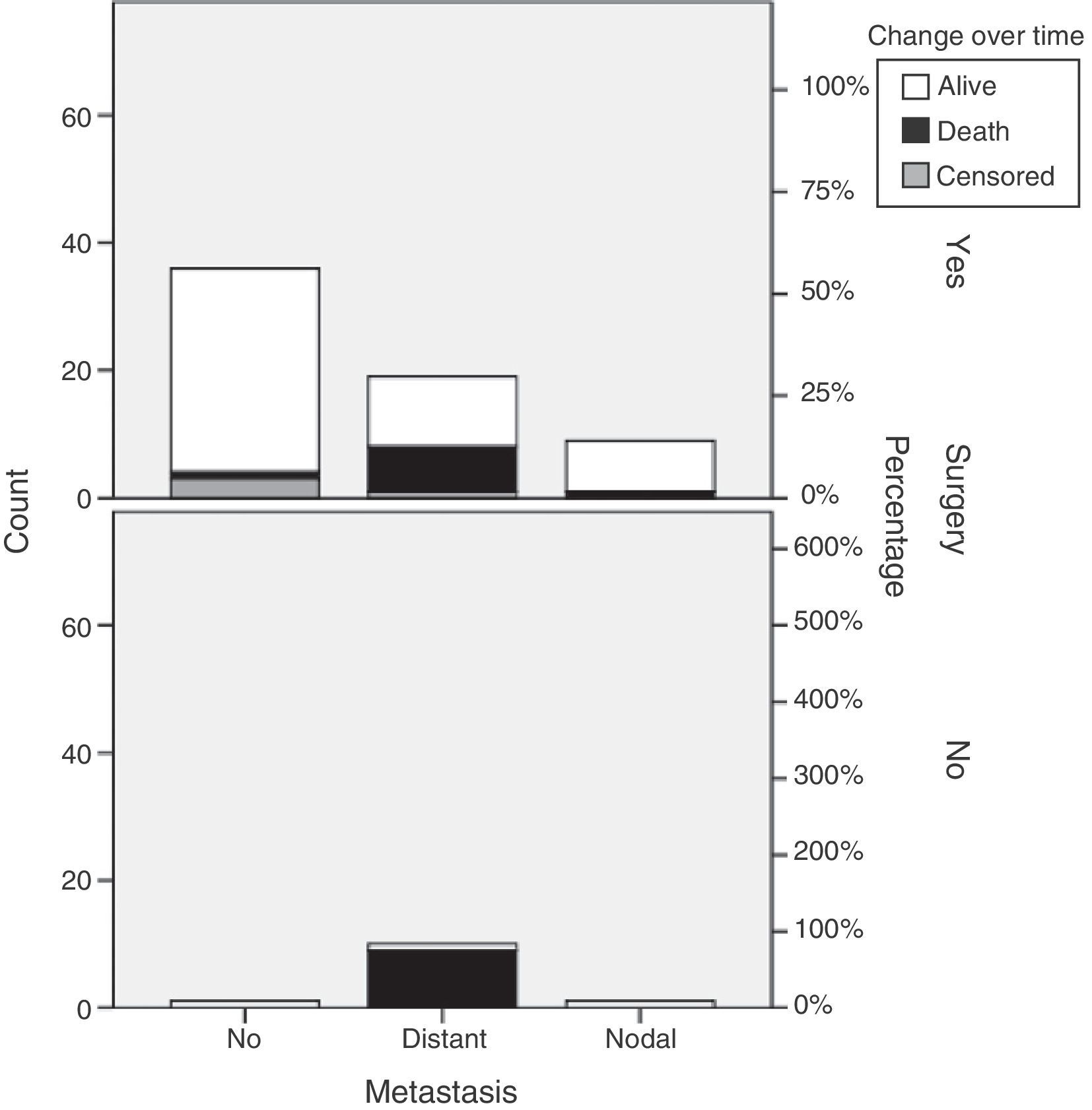
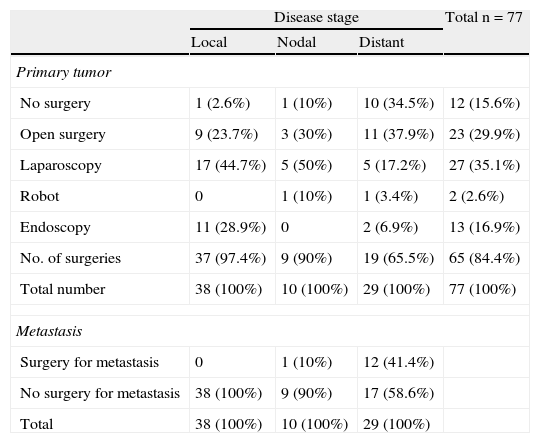
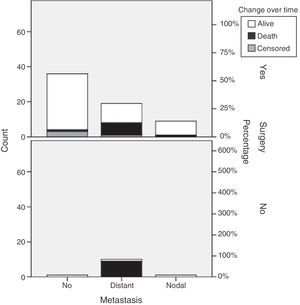
Primary tumor resection was performed in 66 patients (84.6%). Surgery, most commonly laparoscopic, was performed in 97.3% of patients with local disease. Open surgery was mainly performed in 65.5% of patients with distant metastases. Robot-assisted surgery was performed in two patients (Table 3). Thirteen patients from the sample, 12 with distant metastases and one with nodal metastases, underwent surgery for metastatic disease. No chemoembolization or radiofrequency ablation procedures were performed (Table 3).
Surgery.
| Disease stage | Total n=77 | |||
| Local | Nodal | Distant | ||
| Primary tumor | ||||
| No surgery | 1 (2.6%) | 1 (10%) | 10 (34.5%) | 12 (15.6%) |
| Open surgery | 9 (23.7%) | 3 (30%) | 11 (37.9%) | 23 (29.9%) |
| Laparoscopy | 17 (44.7%) | 5 (50%) | 5 (17.2%) | 27 (35.1%) |
| Robot | 0 | 1 (10%) | 1 (3.4%) | 2 (2.6%) |
| Endoscopy | 11 (28.9%) | 0 | 2 (6.9%) | 13 (16.9%) |
| No. of surgeries | 37 (97.4%) | 9 (90%) | 19 (65.5%) | 65 (84.4%) |
| Total number | 38 (100%) | 10 (100%) | 29 (100%) | 77 (100%) |
| Metastasis | ||||
| Surgery for metastasis | 0 | 1 (10%) | 12 (41.4%) | |
| No surgery for metastasis | 38 (100%) | 9 (90%) | 17 (58.6%) | |
| Total | 38 (100%) | 10 (100%) | 29 (100%) | |
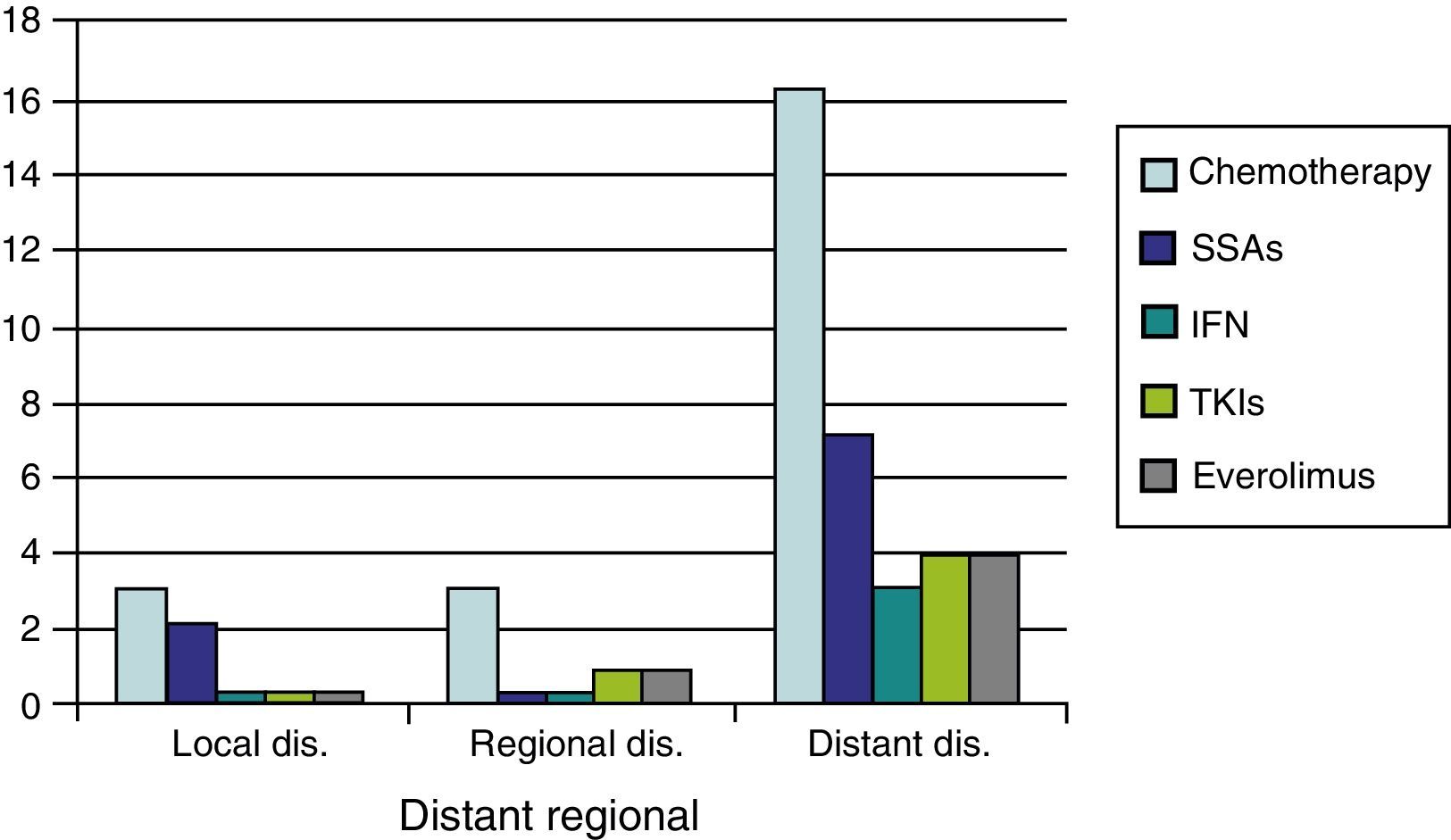
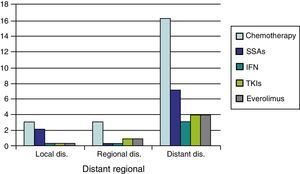
As regards medical treatment, the most commonly prescribed systemic therapies were chemotherapy (CT) (29.5%) and SSAs (12.8%), followed by everolimus (6.4%) TKIs (6.4%), and interferon (3.8%). In patients with distant disease, these proportions were substantially higher (CT 58.6%, SSAs 24.1%, everolimus and TKIs 13.8%, and interferon 10.3%) (Fig. 1).
Seven patients in the study sample (9%) received radiation therapy. Four of these patients (57.1%) had distant metastases, while two patients (28.6%) had no metastases.
As of March 2013, 56 patients (71.8%) are still alive. Complete response to treatment was found in 39 patients (50%), partial response in two patients (2.6%), stabilization in four (5.1%), and progression in 26 patients (33.3%). Eighteen patients (23.1%) died. Four patients were lost to follow-up.
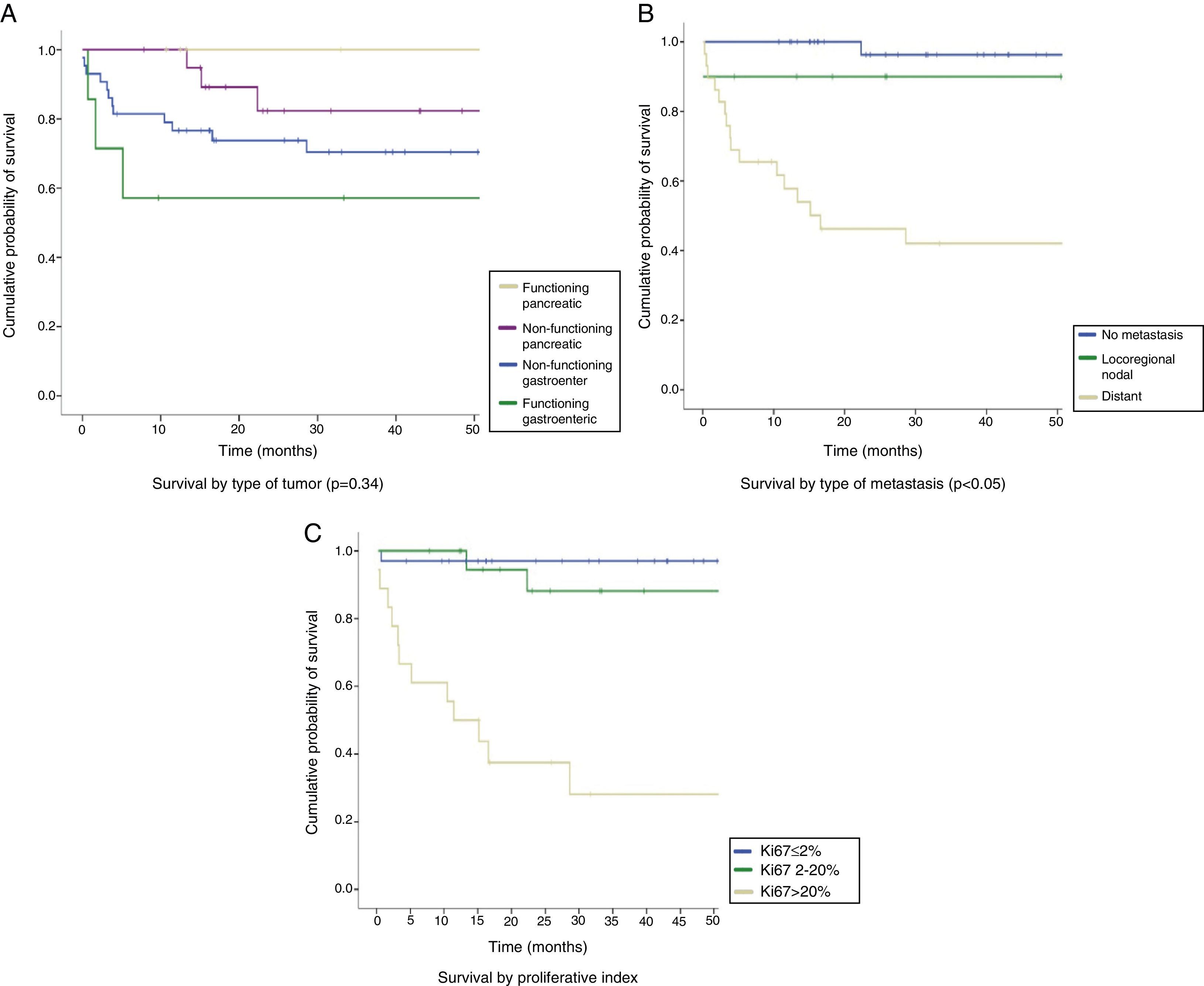
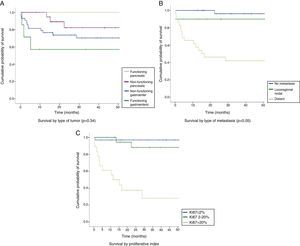
The median follow-up time was 26.7 months (IQR: 13–54.4 months). The overall survival rate of the series was 83.2% at 12 months, 100% in FPNETs, 94.7% in NFPNETs, 76.6% in NFGENETs, and 57.1% in FGENETs. At 24 months, the overall survival rate was 76.8%, 100% in patients with FPNETs and 82.3% in NFPNETs, as compared to 73.8% in NFGENETs and 57.1% in FGENETs (Fig. 2A). These data were not statistically significant (p=0.34). Patients with localized disease had longer survival rates (96.3% at 12 and 24 months) than those with locoregional nodal extension (90% at 12 and 24 months) and distant disease (57.8% at 12 months and 46.2% at 24 months) (Fig. 2B). In turn, WHO grade 3 tumors had poorer prognosis than grade 2 and grade 1 tumors: a 50% 12-month survival rate in grade 3 tumors as compared to 94% and 97% in grade 2 and grade 1 tumors respectively, and a 37.5% 24-month survival rate in grade 3 tumors as compared to 88.15and 97% in grade 2 and grade 1 tumors respectively (p<0.05) (Fig. 2C). Survival in the first 15 months of follow-up was higher in grade 2 tumors because of the death of one patient with a grade 1 NET.
DiscussionHUCSC is a reference hospital for the study of GEP-NETs. Understanding their incidence, the diagnostic and therapeutic approach used at the hospital, and the course of patients is of paramount importance for determining the most adequate management in such cases and enhances the relevance of this study. Comparing our results to those reported by the RGETNE in 20109 allows us to establish the main differences and similarities in an epidemiological setting sharing many characteristics.
Because of the need to rule out associated neoplasms, it should be noted that MEN-1 syndrome had an incidence similar to that in the RGETNE.9 Colorectal cancer was the most prevalent personal history in the sample, being more common in males, according to their epidemiological characteristics.
In agreement with the main series reported, tumors located in the whole gastrointestinal tract were more common than pancreatic tumors; the difference with these was that the most common location was the rectum, instead of the jejunum and ileum. Metastasis from an unknown primary tumor, common in tumors of this type, accounted for only 1.3%, as compared to 9.1% according to the RGETNE.9 Insulinoma was the most common variant among FPNETs, followed by glucagonoma, in contrast to the most important studies reported to date, where gastrinoma was reported to be the second leading tumor. These discrepancies are likely to be related to the relatively small size of the study sample.
The variability in the clinical expression of these tumors was confirmed by this study. Presentation with non-specific gastrointestinal symptoms was more common, and the relevance of an adequate diagnostic procedure that allows for early diagnosis should therefore be emphasized. Despite the low incidence of these tumors, the high percentage of incidental diagnosis warrants their consideration in etiological diagnosis of common gastrointestinal tract conditions such as gastric disease, appendicitis, or colonic polyps, because of differences in therapeutic approach and prognosis.
Although GEP-NETs were traditionally considered to be relatively benign tumors, recent studies have shown that they have a broad malignancy spectrum in which metastatic dissemination of the disease is not uncommon. The high proportion of cases with distant disease again demonstrates the importance of early diagnosis, which may be difficult because of their slow growth.
Considerable disagreement with RGETNE9 is seen as regards the location of metastases, with more frequent nodal than liver involvement. This helps to explain the low frequency of secreting symptoms in carcinoid tumors. Differences also exist in the presence of metastases depending on primary tumor location, being more common in colonic tumors as compared to those located in the jejunum and ileum. The greater frequency of metastases in multiple locations as compared to single metastases should be noted.
The performance of imaging tests is conditioned by tumor variety and different locations. Because of this, and because NFGENETs were most common in the sample, abdominal ultrasound, colonoscopy, and gastroscopy were performed in a high proportion of patients. However, the test most commonly requested was CT because, in addition to its ability to locate the primary tumor, it allows an extension study to be performed. The reviewed studies reported that SRS associated with CT detected virtually 100% of tumors. However, CT was performed for diagnostic purposes in hardly 32% of the patient sample, and was used more frequently for metastasis follow-up and detection. As noted, abdominal ultrasound was performed on a high number of patients because of its availability and low cost, and localized the primary tumor in a small proportion of patients. However, it may be more useful for the detection of liver metastases.
Like in the RGETNE,9 biochemical tests were requested with a frequency lower than expected based on their relevance for NET diagnosis. This may be explained by the high percentage of incidental diagnosis. Serum CgA levels were measured in a higher proportion of patients, because CgA is a general marker, while urinary 5-HIAA levels were tested in a small number of patients, in agreement with the low percentage of carcinoid tumors.
The use of immunohistochemical tests was markedly increased as compared to the RGETNE.9 Thus, CgA, synaptophysin, and Ki-67 were tested in 76.9%, 84.6%, and 91% of cases, as compared to 66%, 50%, and 36%, respectively, in the RGETNE. Calculation of the KI-67 index is of paramount importance to establish tumor grade and decide prognosis and treatment approach. Some of these markers, including CgA, synaptophysin, and NSE, were positive in virtually all samples in which they were tested, which supports the use of immunohistochemistry to confirm diagnosis, unlike biochemical tests, where elevated values were found in only half the cases and the disease could therefore not be ruled out in the presence of levels in the reference range.
Our results show that primary tumor surgery is performed at the HUCSC in the presence of local and regional disease. When distant metastases are found, however, surgery is usually performed on the primary tumor and metastases, and chemotherapy and occasionally SSAs and new therapies are also prescribed. It should be noted that 65.5% of patients with metastatic disease in this series underwent primary tumor surgery, as compared to 44.7% of patients in the RGETNE.9 There is much controversy in the literature about the value of resecting the primary tumor when metastatic disease exists. There is more general agreement on performing debulking in functioning tumors in order to relieve patient symptoms, and greater doubts about its value for patient survival. The data collected appear to suggest that patients who undergo primary tumor surgery in disseminated disease live longer. As this was a retrospective study on a small sample of patients with these characteristics, nothing more should be said (Fig. 3).
In agreement with literature reports, SSAs were administered to control symptoms derived from hormone production and in progressive metastatic disease. In this sample, SSAs were used more frequently for distant disease. New therapies were discontinued in most cases due to disease progression, which may be related to their administration in advanced stages. Future studies to ascertain the value of these therapeutic measures in earlier disease stages will therefore be of great interest.
The overall prognosis was favorable, with a two-year survival rate of 76.8%, although the follow-up period was not long enough to rule out late events, as these are slowly growing tumors. Greater survival was seen in women, localized disease, and WHO grade 1, because the death of the patient with grade 1 NET in the first five months was due to infectious complications after surgery. It may therefore be concluded that, despite the small sample size, these results are similar to those from other larger series previously reported.
Because of the relatively low prevalence of NETs, and the diversity of their distribution and presentation, collaborative studies between centers are needed to allow for the standardization of diagnostic and therapeutic management, thus promoting the development of protocols which will contribute to improving survival rates. In this regard, units specialized in NETs such as the one operating at the HUCSC facilitate data collection and allow for the objective evaluation of care activities.
Conflicts of interestDr. de Miguel and Drs. Díaz, Satre y Ortega have taken part on various occasions in training courses and scientific research with Pfizer, Novartis e Ipsen.
Please cite this article as: de Miguel Novoa MP, Fernández Capel F, Redondo Sedano JV, Sellers Carrera M, Aranda Jiménez V, Ortiz Pereira P, et al. Tumores neuroendocrinos gastroenteropancreáticos: características clínicas, proceso diagnóstico y pronóstico en el Hospital Universitario Clínico San Carlos (Madrid). Endocrinol Nutr. 2014;61:234–241.