Differentiated thyroid carcinoma staging is increasingly important due to the current trends to a less intensive therapy in low-risk patients. The TNM system is most widely used, but other systems based on follow-up of several patient cohorts have been developed. When these systems have been applied to other populations, results have been discordant.
Our study evaluates the suitability of several differentiated thyroid carcinoma staging systems in a Spanish population.
Material and method729 patients with differentiated thyroid carcinoma and staging data available were enrolled. Mean follow-up time was 10.8 years.
The TNM, EORTC, AMES, Clinical class, MACIS, Ohio, NTCTCS, and Spanish systems were applied to all histological types. The Kaplan–Meier survival curves for each system were analyzed, and compared using the proportion of explained variation (PEV).
ResultsThe demographic and clinical characteristics of our population were similar to those of other Spanish and international cohorts reported.
The best systems were NTCTCS, with 74.7% PEV, and TNM (68.3%), followed by the Ohio, MACIS, EORTC, and AMES systems with minimal differences between them, while the least adequate were the Spanish (55.2%) and Clinical class (47.1%) systems.
ConclusionThe NTCTCS staging system was found to be better than TNM in our population but, because of its simplicity and greater dissemination, the TNM appears to be recommended for staging of patients with differentiated thyroid carcinoma.
La estadificación del carcinoma diferenciado de tiroides cobra gran importancia ante la tendencia actual de reservar los tratamientos más intensivos a los casos de peor pronóstico. Aunque el más difundido es el basado en la clasificación TNM, se han desarrollado otros a partir de seguimiento de cohortes de uno o varios centros, pero al aplicarlos en otras poblaciones los resultados han sido discordantes.
El objetivo de este estudio es evaluar la adecuación de varios sistemas de estadificación del carcinoma de tiroides a una población española.
Material y métodoSe incluyeron 729 pacientes diagnosticados de carcinoma diferenciado de tiroides, de los que se disponía de todos los datos necesarios para la estadificación por los sistemas estudiados, seguidos durante una media de 10,8 años.
Se estudiaron los sistemas TNM, EORTC, AMES, Clase clínica, MACIS, Ohio, NTCTCS y español, aplicados a todos los tipos histológicos. Se analizaron las curvas de Kaplan–Meier para cada sistema y la comparación entre ellos se realizó mediante la proporción de varianza explicada.
ResultadosLas características demográficas y clínicas de nuestra población son similares a las de otras cohortes españolas e internacionales publicadas.
Los mejores sistemas fueron NTCTCS, con una proporción de varianza explicada del 74,7% y TNM (68,3%), seguidos por Ohio, MACIS, EORTC y AMES con mínimas diferencias entre ellos, y los menos adecuados el español (55,2%) y Clase clínica (47,1%).
ConclusiónPese a que NTCTCS ha resultado mejor, por su sencillez y difusión parece recomendable usar el TNM para los pacientes con carcinoma diferenciado de tiroides.
Differentiated thyroid carcinoma (DTC) is the most common malignant endocrine tumor, and its incidence has been increasing in recent years, not only at the expense of microcarcinomas detected as the result of improved diagnostic procedures, but also because of an actual increase in the frequency of large tumors.1,2
DTC is usually poorly aggressive, with a high complete remission rate and a 10-year mortality rate of less than 10%. However, some cases have an unfavorable course, with local, nodal, or distant recurrence which decreases the quality of life of patients and may result in death.
To allow for the early detection of these cases requiring more intensive monitoring and treatment, and to avoid the inadequate use of resources and excess treatment of patients who do not need it, several systems which stage the risk of death have been established. The most widely used staging system is the one based on the TNM classification of the American Joint Commission on Cancer, but other systems based on the monitoring of large patient cohorts from one or several centers have been developed. These systems are adequate for the populations where they were developed, but their predictive value decreases and their results are discordant when applied to other populations.
The primary objective of this study was to assess the suitability of various staging systems for thyroid carcinoma in a Spanish population.
The secondary objective was to define the demographic, clinical, and evolutionary characteristics of a cohort of patients with differentiated thyroid carcinoma.
Material and methodsA retrospective study was conducted on patients treated for differentiated thyroid carcinoma at Hospital General Universitario Gregorio Marañón between 1970 and 2013. Patient inclusion criteria were as follows:
- •
Initial surgical treatment.
- •
Histologically confirmed diagnosis of differentiated thyroid carcinoma.
- •
Clinical follow-up for at least one year, unless the patient died during this period.
- •
Availability of clinical and pathological data required for staging using the methods analyzed:
- -
Patient age and sex at the time of diagnosis.
- -
Extent of surgery performed.
- -
Histological type of tumor, and variant if applicable.
- -
Size of primary tumor.
- -
Tumor extension to perithyroid tissues.
- -
Presence of regional adenopathies.
- -
Presence of distant metastases at diagnosis.
- -
A total of 1152 clinical records were reviewed, of which 423 were excluded due to non-compliance with any of the inclusion criteria. The main reason for exclusion was the lack of any of the data required for staging.
The status of the 729 patients enrolled at study closure (May 2013) was defined as:
- •
Remission (patient alive and with no clinical, radiographic, or laboratory evidence of persistent or recurrent tumor).
- •
Patient alive with persistent disease.
- •
Death from the tumor.
- •
Death from other causes.
- •
Patient lost to follow-up.
The clinical management of these patients was according to standard practice at our hospital during these years: most patients underwent total thyroidectomy with or without lymphadenectomy depending on findings in the surgical field and on the usual practice of the surgeon, ablation of thyroid remnants with 131I, and long-term treatment with levothyroxine in TSH-suppressing doses. However, the number of patients given less intensive treatment, in accordance with the most recent clinical practice guidelines, increased in the final years of the study. Treatment with conventional chemotherapy, tyrosine kinase inhibitors, or external radiotherapy was used in isolated cases.
The staging systems evaluated included: TNM 6th edition (2002), the European Organization for Research and Treatment of Cancer (EORTC),3 AMES,4 Clinical class,5 Metastasis, Age, Completeness of resection, Invasion and Size (MACIS),6 Ohio,7 the National Thyroid Cancer Treatment Cooperative Study Registry Group (NTCTCS),8 and Spanish.9 Although the Clinical class, MACIS, and Spanish staging systems were developed on patient cohorts with papillary carcinoma only, in this study they were applied to the other types. Table 1 summarizes the characteristics of these systems.
Staging systems.
| TNM |
| T1: ≤2cm |
| T2: 2–4cm |
| T3: >4cm or small local extension |
| T4a: significant local extension |
| T4b: extension to spine or major vessels |
| N0: no adenopathies |
| N1a: cervical adenopathies |
| N1b: mediastinal adenopathies |
| M0: no metastases |
| M1: distant metastases or non-regional adenopathies |
| Patients younger than 45 years |
| Stage I: any T, any N, M0 |
| Stage II: any T or N, M1 |
| Patients older than 45 years |
| Stage I: T1, N0, M0 |
| Stage II: T2, N0, M0 |
| Stage III: T1-3, N0-1a, M0 |
| Stage IV: T1-4a, N0-1b, M0 |
| Stage IV: T4b, any N, M0 |
| Stage IV-C: any T and N, M1 |
| Medullary: all patients older than 45 years |
| Anaplastic: all stage IV |
| EORTC3 |
| Age (years)+12 if ♂+10 if extrathyroid extension+x+y |
| x: 10 if medullary or poorly differentiated follicular, 45 if anaplastic |
| y: 15 if distant metastases, 30 if multiple metastases |
| Stage I: <50 |
| Stage II: 50–65 |
| Stage III: 66–83 |
| Stage IV: 84–108 |
| Stage IV: >108 |
| AMES4 |
| Low risk: patients<40 (males) or 50 years (females) with no metastases. In older patients, those whose tumor is <5cm, is intrathyroid if papillary or with minimal capsular invasion if follicular, and with no metastases |
| High risk: patients with distant metastases. In older patients, those not meeting any of the conditions considered low risk. |
| Clinical class5 |
| Class I: intrathyroid |
| Clase II: regional adenopathies |
| Clase III: extrathyroid (unresectable tumor or adenopathies) |
| Class: distant metastases |
| MACIS6 |
| (Age×0.08 or 3.1 in <40 years)+(0.3×size in cm)+1 (if incomplete resection)+1 (if local invasion)+3 (if metastases) |
| Stage I: <6 |
| Stage II: 6–7 |
| Stage III: 7–8 |
| Stage IV: >8 |
| Ohio7 | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|
| Size | <1.5cm | 1.5–4.4 | ≥4.5 | Any |
| Adenopathies | No | Yes | Any | Any |
| Local invasion | No | No | Yes | Any |
| Metastasis | No | No | No | Yes |
| NTCTCS8 |
| Size: <1cm/1–4/>4cm |
| Age: ≤45 years/>45 |
| Histological type |
| Multifocality: |
| Micro/macroscopic |
| Intra/extrathyroid |
| Tumor differentiation |
| Metastasis: cervical/extracervical |
| Age | Papillary | Follicular | Medullary | ||
|---|---|---|---|---|---|
| <45 | ≥45 | <45 | ≥45 | ||
| Size of primary tumor (cm) | |||||
| <1 | I | I | I | II | Hyperplasia I<1cm II>1cm or cervical adenopathies IIIExtrathyroid or extracervical metastases IV |
| 1–4 | I | II | I | III | |
| >4 | II | III | II | III | |
| Multifocality | |||||
| Microscopic | I | II | I | III | AnaplasticAll IV |
| Gross or capsular | I | II | II | III | |
| Microscopic extrathyroid | I | II | I | III | |
| Gross extrathyroid | II | III | II | III | |
| Poorly differentiated | NA | NA | III | III | |
| Metastasis | |||||
| Cervical adenopathies | I | III | I | III | |
| Extracervical | III | IV | III | IV | |
| Spanish9 |
| Age: one point if <50 years, 2 points if ≥50 years |
| Size: one point if ≤4cm, 2 points if >4cm |
| Extension: one point if intrathyroid and 2 points if extrathyroid |
| Histological variant: |
| One point for the classical, follicular, and diffuse sclerosing variants |
| Two points for the solid and tall cell variants |
| Three points for the poorly differentiated variant (high mitosis and atypia rates, necrotic areas) |
| Prognostic index: (3×age)+(2×size)+(6×extension)+(2×histological variant) |
| Risk stages: |
| Low: <18 points |
| Intermediate: 18–22 points |
| High: >22 points |
PVE: proportion of variance explained.
Qualitative variables are given with their frequency distribution. Quantitative variables are given as mean, standard deviation (SD), and range. Distribution of the variables was verified against theoretical models in all cases, and in the event of asymmetry, the median and its interquartile range were calculated.
The association between qualitative variables was assessed using a Pearson's Chi-square test (χ2) or a Fisher's exact test if more than 25% of the expected cases were less than 5. For quantitative variables, a Student's t test was used for normally distributed variables; otherwise, a Mann–Whitney U test was used.
An analysis of Kaplan–Meier curves was performed to study the relationship of the different variables with mortality. To compare the different staging systems in the study population, the proportion of variance explained (PVE) by each of them was used according to the Royston and Sauerbrei method.10 This statistical parameter provides the proportion of variance of the study variable, in this case tumor-specific mortality, explained by each predictive system; higher values reflect a greater predictive power and, thus, a better suitability of the system for the study population.
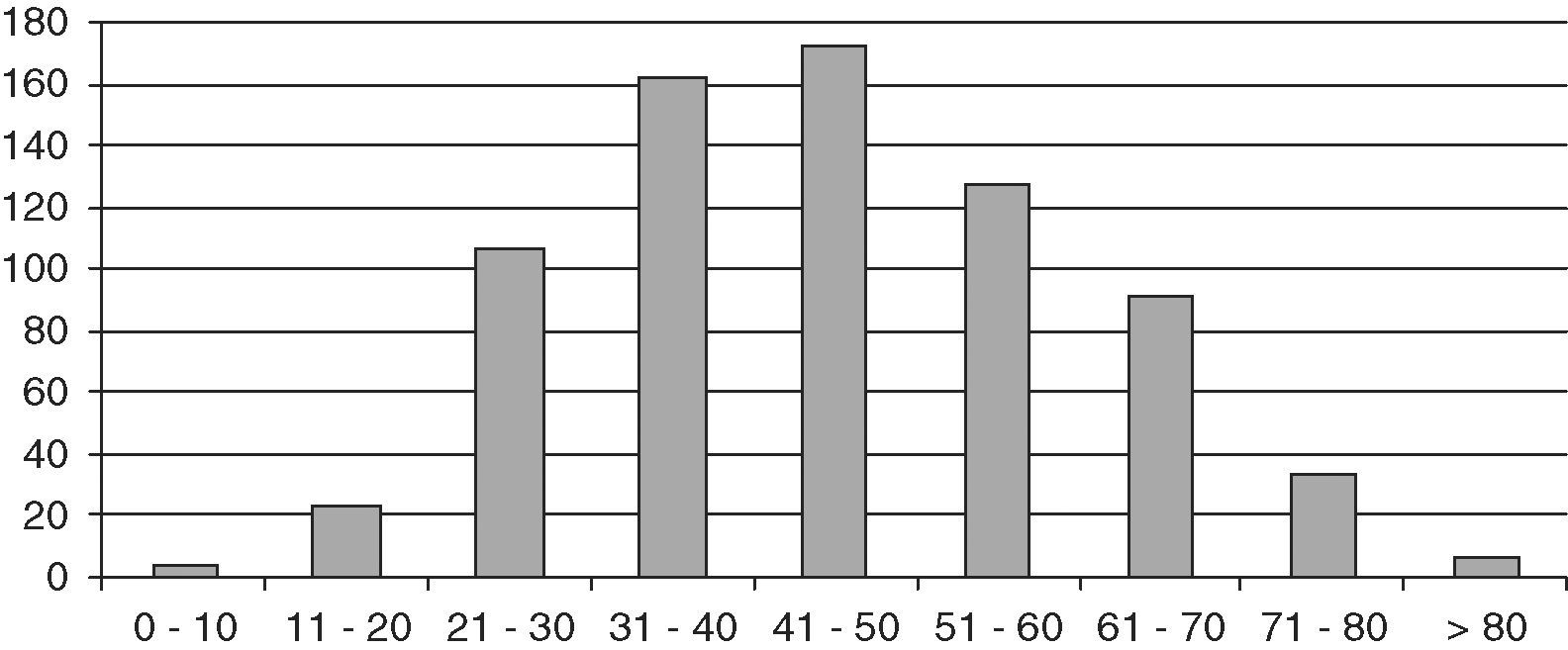
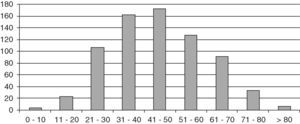
ResultsThe study population consisted of 729 patients, 145 males and 584 females, with a mean follow-up time of 10.8 years (SD 8.4; range 2 months–42 years), which represented a follow-up of 8184 patient/years. Mean age at diagnosis was 45.2 years (SD 15.4; range 6–87). Fig. 1 shows distribution by age.
Seventy-two patients (20 males and 52 females) were lost to follow-up and were not included in the survival study. In these patients, final status was defined as status at the last valid review. Fifty-nine patients (9%) died, 21 of them (3.2%) due to thyroid carcinoma.
The most common histological type was papillary carcinoma, found in 588 patients (80.7%), with a predominance of the classical (442) and follicular (100) variants; 108 patients had follicular carcinoma, 29 Hürthle cell carcinoma, and 4 insular carcinoma. Mean age at diagnosis was significantly lower in papillary carcinoma (42.8 years; SD 17.3) as compared to follicular carcinoma (46.6; SD 15.8, p<0.05) and Hürthle cell carcinoma (51.8; SD 14.3, p<0.005); the difference between follicular and Hürthle cell carcinoma was not significant.
Mean tumor size was 2.26cm (SD 1.74), and size was significantly greater in males (2.7; SD 2.05) as compared to females (2.13; SD 1.63, p<0.005), and in follicular (3.39; SD 2.01) and Hürthle cell (3.8; SD 2.13) carcinomas versus papillary carcinomas (1.9; SD 1.5; p<0.001). No significant differences were found by age at diagnosis. However, a gradual decrease in size was seen during the study time from 3.38cm (SD 1.88) in patients diagnosed in the 1970–1981 period to 2cm (SD 1.58, p<0.005) from 2002.
Variables significantly associated with overall mortality included age at diagnosis, the presence of other malignant tumors, the presence of metastasis from thyroid carcinoma at diagnosis, and thyroglobulin levels at the first laboratory control tests after surgery.
Death from thyroid carcinoma was also associated with tumor size, extrathyroid tumor extension at surgery, histological type (more common in follicular tumors), and local, nodal, or distant recurrence. A multivariate analysis with all study variables could not be performed because of the low number of deaths. Analysis was therefore performed in several steps, age at diagnosis and maximum tumor size being maintained as fixed variables because they showed the greatest significance level in the initial univariate analysis.
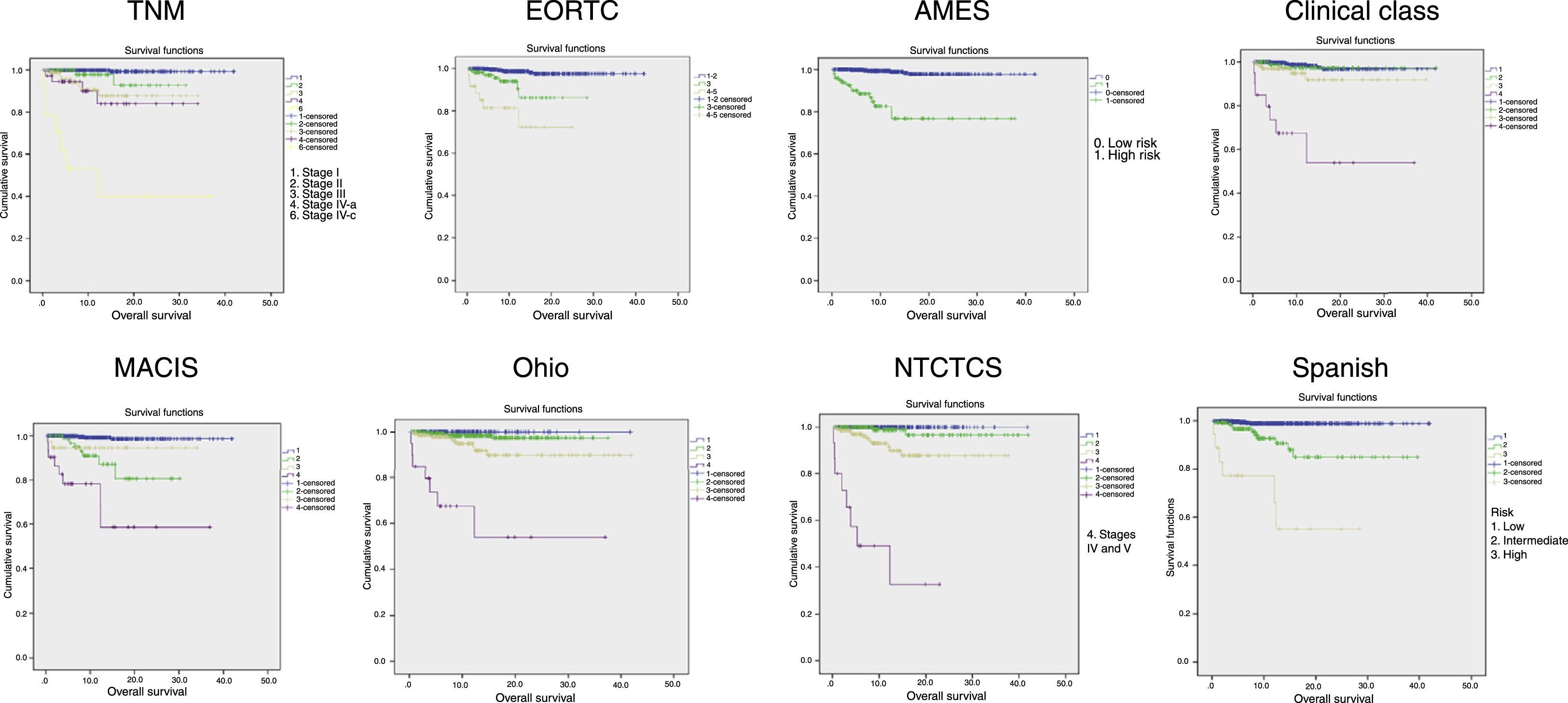
Fig. 2 shows the Kaplan–Meier curves of tumor-specific survival for each system. They all show a clear correlation between tumor stage and mortality, particularly at the expense of differences between extreme stages, while intermediate stages are closer to the lowest stage.
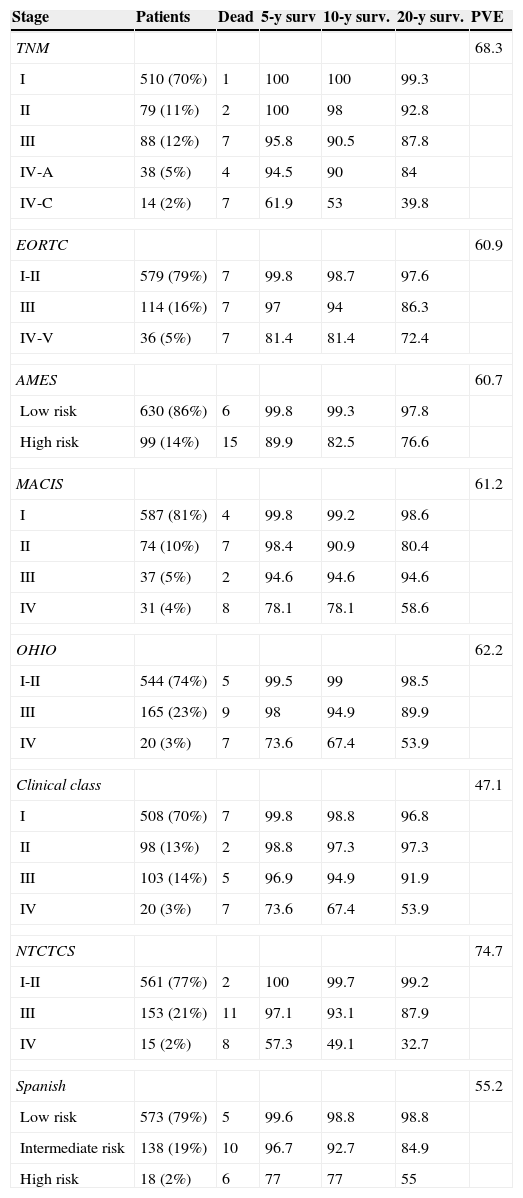
Table 2 shows patient distribution in the different stages, survival at 5, 10, and 20 years, and PVE for each system. In the EORTC, Ohio, and NTCTCS systems, stages I and II were pooled because no death occurred in stage I patients, while in the EORTC system, stages IV and V were pooled because there were only three patients in the latter stage. The greatest PVE was found for the NTCTCS system (74.7%), followed by the TNM system (68.3%). Minimal differences were seen between the other systems, with PVEs of approximately 60%, except for the Spanish system (55.2%) and, especially, the Clinical class, with a PVE of 47.1% only.
Staging systems. Patients included in each stage, 5, 10, and 20-year survival, and proportion of variance explained.
| Stage | Patients | Dead | 5-y surv | 10-y surv. | 20-y surv. | PVE |
|---|---|---|---|---|---|---|
| TNM | 68.3 | |||||
| I | 510 (70%) | 1 | 100 | 100 | 99.3 | |
| II | 79 (11%) | 2 | 100 | 98 | 92.8 | |
| III | 88 (12%) | 7 | 95.8 | 90.5 | 87.8 | |
| IV-A | 38 (5%) | 4 | 94.5 | 90 | 84 | |
| IV-C | 14 (2%) | 7 | 61.9 | 53 | 39.8 | |
| EORTC | 60.9 | |||||
| I-II | 579 (79%) | 7 | 99.8 | 98.7 | 97.6 | |
| III | 114 (16%) | 7 | 97 | 94 | 86.3 | |
| IV-V | 36 (5%) | 7 | 81.4 | 81.4 | 72.4 | |
| AMES | 60.7 | |||||
| Low risk | 630 (86%) | 6 | 99.8 | 99.3 | 97.8 | |
| High risk | 99 (14%) | 15 | 89.9 | 82.5 | 76.6 | |
| MACIS | 61.2 | |||||
| I | 587 (81%) | 4 | 99.8 | 99.2 | 98.6 | |
| II | 74 (10%) | 7 | 98.4 | 90.9 | 80.4 | |
| III | 37 (5%) | 2 | 94.6 | 94.6 | 94.6 | |
| IV | 31 (4%) | 8 | 78.1 | 78.1 | 58.6 | |
| OHIO | 62.2 | |||||
| I-II | 544 (74%) | 5 | 99.5 | 99 | 98.5 | |
| III | 165 (23%) | 9 | 98 | 94.9 | 89.9 | |
| IV | 20 (3%) | 7 | 73.6 | 67.4 | 53.9 | |
| Clinical class | 47.1 | |||||
| I | 508 (70%) | 7 | 99.8 | 98.8 | 96.8 | |
| II | 98 (13%) | 2 | 98.8 | 97.3 | 97.3 | |
| III | 103 (14%) | 5 | 96.9 | 94.9 | 91.9 | |
| IV | 20 (3%) | 7 | 73.6 | 67.4 | 53.9 | |
| NTCTCS | 74.7 | |||||
| I-II | 561 (77%) | 2 | 100 | 99.7 | 99.2 | |
| III | 153 (21%) | 11 | 97.1 | 93.1 | 87.9 | |
| IV | 15 (2%) | 8 | 57.3 | 49.1 | 32.7 | |
| Spanish | 55.2 | |||||
| Low risk | 573 (79%) | 5 | 99.6 | 98.8 | 98.8 | |
| Intermediate risk | 138 (19%) | 10 | 96.7 | 92.7 | 84.9 | |
| High risk | 18 (2%) | 6 | 77 | 77 | 55 | |
PVE: proportion of variance explained.
The purpose of malignant tumor staging is to select the most adequate treatment for each case and to make a tentative prognosis. It also allows for comparing the results of different hospitals or treatments. The adequate staging of differentiated thyroid carcinoma is becoming increasingly important because current clinical guidelines11–13 advocate treatments less intensive than those traditionally recommended in low risk tumors, which represent the majority of cases of thyroid carcinoma.
The TNM classification is the one most widely used. It is also a simple system, and is therefore becoming something like a “common language” in thyroid cancer. However, there are some deficiencies associated with it, particularly the fact that age is considered as a categorical, rather than a continuous, variable, with an arbitrary cut-off point that coincides with the mean age of most cohorts published, as occurred in our study. Because of this, with a minimum time difference a same case may change from a low stage to a much more severe stage. Thus, Tran Cao et al.14 criticized this distinction by pointing out that in patients under 45 years of age, mortality is 11 times greater in stage II as compared to stage I, while no differences exist in older patients, and concluded that the current classification underestimates the prognostic significance of metastases in the younger population.
All the other systems analyzed either do not include age as a variable (Clinical class, Ohio) or include it as a categorical variable with a similar cut-off point, except for the MACIS and EORTC systems, which include age as a continuous variable. In the EORTC system, age is the factor with the greatest specific weight in the prognostic equation, probably because the target established was overall rather than tumor-specific mortality.
An additional problem common to all systems is that they include variables which are only available after thyroidectomy and which are therefore not helpful for planning the initial surgical approach.
When these systems are compared in populations different from those in which they were developed, the results are highly variable, although the TNM classification is usually the most useful.15–18 Wong et al.19 recently developed the Cedars-Sinai Medical Center (CSMC) staging system. A comparison of this new staging system with the previous ones did not find any of them to be clearly superior to the others.
Two comparisons have been published in Spain. In one of them, Gómez Arnáiz et al.20 only found significant differences after combining the intermediate stages of each system with the stage with the poorest prognosis in the case of the EORTC system or with the best prognosis in the cases of the TNM and Clinical class systems, and concluded that, for clinical purposes, it is more helpful to categorize patients as low or high risk. Donnay et al.21 subsequently reported that the TNM classification is the best model for predicting recurrence, while the ETA classification is the best predictor of tumor death or persistence. However, their report did not mention any other systems.
The system with the greatest PVE in our study was the NTCTCS system, which showed a small difference compared to TNM and considerable differences compared to all the other systems analyzed. The Spanish system was among those with a poorest association (only the Clinical class showed worse results), which is surprising since it was developed in a population of a similar origin.
The main limitations of this study are the high proportion of patients excluded (36.7% of the initial sample), mainly because the data required for adequate staging were not available, and due to the proportion of cases lost to follow-up, almost 10% of the patients enrolled. However, this represents the largest cohort of patients with DTC reported in Spain, and the one with the longest mean follow-up time after the joint series of two hospitals reported by Reverter et al.22
Although several physicians were involved in the diagnosis and treatment of these patients during this period, the management protocol remained constant in general, and differences between the early and late years were due to improvements in diagnostic procedures, with the introduction of thyroid ultrasound examination and ultrasound-guided FNA to assess thyroid nodules.
An additional limitation, inherent to the study duration, lies in the fact that measurements of TSH, thyroglobulin, and thyroglobulin antibodies were not available until 1981. The baseline levels of these parameters in the 45 cases diagnosed before that date are therefore unknown, and the results collected may not be equivalent to current values with the different test procedures used during this period, as the specifications of prior procedures are not known.
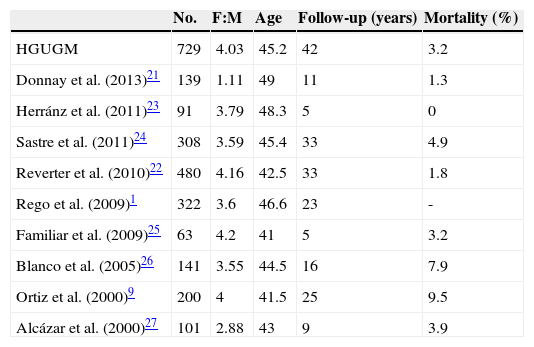
The clinical and demographic characteristics of this cohort were similar to those reported in other Spanish series1,9,21–27 (Table 3), except for the lower female:male ratio found by Donnay et al.21, and in international studies.2,19,28–32 Tumor-specific mortality was also similar to the reported rates. A direct comparison of crude mortality rates is however not possible because in a tumor such as DTC, with a very low and very long-term mortality, potential differences in mortality should be assessed in established time periods, using Kaplan–Meier curves to analyze survival.
Comparison with other Spanish series.
| No. | F:M | Age | Follow-up (years) | Mortality (%) | |
|---|---|---|---|---|---|
| HGUGM | 729 | 4.03 | 45.2 | 42 | 3.2 |
| Donnay et al. (2013)21 | 139 | 1.11 | 49 | 11 | 1.3 |
| Herránz et al. (2011)23 | 91 | 3.79 | 48.3 | 5 | 0 |
| Sastre et al. (2011)24 | 308 | 3.59 | 45.4 | 33 | 4.9 |
| Reverter et al. (2010)22 | 480 | 4.16 | 42.5 | 33 | 1.8 |
| Rego et al. (2009)1 | 322 | 3.6 | 46.6 | 23 | - |
| Familiar et al. (2009)25 | 63 | 4.2 | 41 | 5 | 3.2 |
| Blanco et al. (2005)26 | 141 | 3.55 | 44.5 | 16 | 7.9 |
| Ortiz et al. (2000)9 | 200 | 4 | 41.5 | 25 | 9.5 |
| Alcázar et al. (2000)27 | 101 | 2.88 | 43 | 9 | 3.9 |
F:M: female:male ratio.
In conclusion, although the NTCTCS system was the one best fitted to our population because of its simplicity and wide dissemination, the TNM classification would appear to be adequate for staging patients with DTC.
We need to develop a prediction system which is independent of the result of the histological study of the surgical specimen, including radiographic, cytological, or genetic variables, to allow for the adequate planning of the extent of thyroidectomy.
Conflicts of interestThe authors have no conflicts of interest in relation to this article.
We thank Professor Antonino Jara, emeritus Head of the Department of Endocrinology and Nutrition of the HGUGM, for his continuous support for the conduct of this study and his critical review of the original; Dr. Angel Bittini, from the Department of Nuclear Medicine, for the collection of patient data over decades, excellent data management, and the transfer of this data; José María Bellón, from the Health Research Institute of Hospital Gregorio Marañón, for collaboration in statistical data processing; and to all our colleagues who have monitored and treated the study patients during this period.
Please cite this article as: Andía Melero VM, Martín de Santa-Olalla Llanes M, Sambo Salas M, Percovich Hualpa JC, Motilla de la Cámara M, Collado Yurrita L. Comparación de sistemas de estadificación del carcinoma diferenciado de tiroides en una población española. Endocrinol Nutr. 2015;62:152–160.