Gestational diabetes mellitus (GDM) is associated to an increased risk of pregnancy-induced hypertension (PIH). Ambulatory blood pressure monitoring (ABPM) has been used to detect PIH and preeclampsia, but few data are currently available on its use in women with GDM. The aim of this study was to achieve early identification in women with GDM of BP profiles (detected by ABPM) that could define a population at greater risk of developing PIH and preeclampsia.
Material and methodsA prospective study of 93 normotensive women with GDM in whom 24-h ABPM was performed (using a Spacelabs 90207 monitor) at 28–32 weeks of pregnancy. Clinical and laboratory variable and obstetric and perinatal outcomes were analyzed.
ResultsMean age was 34.8±4.39years, and 5.4% of patients developed PIH. Higher levels of HbA1c (p=0.005) and microalbumin (p=0.001) were seen in patients with PIH. Patients with non-dipper patterns (50.5%) had higher values of night-time systolic BP (106.7 vs. 98.4mmHg) and night-time diastolic BP (64.8 vs. 57.2mmHg) (p<0.001). Lower birth weights (3084.57 vs. 3323.7) (p=0.021) and shorter gestational age at delivery (38.67 vs. 39.27 weeks) (p=0.04) were found in women with non-dipper pattern. High night-time systolic BP significantly increased the chance of developing PIH (OR: 1.18; 95%CI: 1.00–1.39; p=0.043).
ConclusionsPatients with GDM have BP changes, with predominance of the non-dipper pattern and higher night-time systolic and diastolic BP, changes that could be useful predictors of PIH. High night-time systolic BP values increase the risk of developing PIH. Further studies are needed to ascertain the relationships between BP changes and obstetric and perinatal complications.
Las pacientes con diabetes mellitus gestacional (DMG) presentan un mayor riesgo de desarrollar hipertensión arterial inducida por el embarazo (HIE). La monitorización ambulatoria de presión arterial (MAPA) ha sido usada para detectar HIE y preeclampsia, pero hasta la fecha no ha sido suficientemente estudiada en DMG. El objetivo del presente trabajo es identificar de forma precoz, en mujeres con DMG, perfiles de presión arterial (PA), detectados mediante MAPA, que pudieran definir una población de mayor riesgo de desarrollar HIE y preeclampsia.
Material y métodosEstudio prospectivo en 93 pacientes con PA normal con DMG. Se les implantó entre la semana 28-32 de gestación la MAPA durante 24h (Spacelabs 90207) y se analizaron variables clínicas, analíticas y resultados obstétricos y perinatales.
ResultadosLa edad media fue 34,8±4,39años. Cinco pacientes (5,4%) desarrollaron HIE. Encontramos niveles más elevados de HbA1c (p=0,005) y microalbuminuria (p=0,001) entre las que desarrollaron HIE. Las pacientes con patrón no dipper (50,5%) presentaron cifras de PAS nocturna (106,7 vs 98,4mmHg) y PAD nocturna (64,8 vs 57,2mmHg) más elevadas (p<0,001). Se observó menor peso al nacimiento (3.084,57 vs 3.323,7; p=0,021) y menor semana de gestación en el momento del parto (38,67 vs 39,27 semanas; p=0,04) en pacientes con patrón no dipper respecto al dipper. La PAS nocturna elevada se asoció con un incremento significativo de la probabilidad de HIE (OR: 1,18; IC95%: 1,00-1,39; p=0,043).
ConclusionesEn la DMG existen alteraciones tensionales con un predominio de patrón no dipper de PA y con valores más elevados de PAS y PAD nocturnos, pudiendo ser estas alteraciones predictoras de HIE. Los valores elevados de PAS nocturna aumentan el riesgo de desarrollo de HIE. Se requieren futuros estudios para determinar la relación entre las alteraciones tensionales y las complicaciones maternas y perinatales.
Gestational diabetes mellitus (GDM) is defined as hyperglycemia diagnosed in the second or third trimester of pregnancy and with no clear evidence of prior diabetes mellitus (DM).1 It is estimated that GDM affects 8% of all pregnant women in Spain.2 Hyperglycemia during pregnancy can result in fetal complications (e.g., macrosomia), complications at delivery (shoulder dystocia or the need for cesarean section, etc.), or maternal complications (e.g., preeclampsia).3
Pregnancy-induced hypertension (PIH) affects 5–10% of all pregnancies4 and includes gestational hypertension, preeclampsia, eclampsia and chronic hypertension.5 As in GDM,6 PIH implies an increased risk of developing cardiovascular disease (CVD)7 and maternal and perinatal morbidity.4 Despite the fact that PIH is 2–3 times more common in patients with GDM,8 few studies have evaluated early detection methods, allowing for early treatment and therefore a lessened impact upon pregnancy.
Ambulatory blood pressure monitoring (ABPM) allows for measurements to be made over 24–48h and for circadian rhythm analysis,9 since blood pressure (BP) reaches a minimum during the first hours of sleep and increases in the early morning, a difference of 10–20% being considered normal (dipper pattern). Nocturnal pressure reductions of less than 10% define the so-called non-dipper pattern.10 Many studies have attempted to determine whether ABPM could be a useful tool for predicting early changes in BP circadian rhythm in patients who subsequently develop PIH. In fact, some studies in pregnant women with type 1 DM11 have shown an increase in the frequency of the non-dipper pattern in the second trimester of pregnancy to be predictive of the development of PIH.
The objective of our study was to establish whether certain BP profiles (as identified by ABPM) in GDM can identify a population at risk of developing PIH and preeclampsia, and therefore allow for the adoption of early preventive actions aimed at reducing maternal and perinatal complications.
Material and methodsA prospective study was carried out in patients with GDM and normal BP seen at the Endocrinology and Pregnancy clinic of Hospital Universitario Puerta del Mar (Cádiz, Spain) to analyze the presence of BP changes detected by 24-h ABPM and their potential evolution toward PIH. The correlation to inflammatory and clinical parameters and obstetric and perinatal complications was also explored. The study period was from August 2014 to August 2017.
The following inclusion criteria were established: women presenting normal BP (systolic BP [SBP] ≤130mmHg, diastolic BP [DBP] ≤80mmHg evidenced by point measurements on an outpatient or ambulatory basis) with physiological gestation and diagnosed with GDM following a pathological O'Sullivan test (100g oral glucose tolerance test [OGTT]) yielding two abnormally high values and taking 105, 190, 165 and 145mg/dl (basal and after 60, 120 and 180min, respectively) as reference values. The exclusion criteria were: women with chronic hypertension or SBP >130 or DBP >80mmHg evidenced by point measurements on an outpatient or ambulatory basis, or treated with antihypertensive drugs, with a diagnosis of placental insufficiency, pregestational or monogenic diabetes, as well as morbid obesity (body mass index [BMI] >40kg/m2), underlying chronic systemic disease, acute infectious disease, smoking, or lack of informed consent.
At study entry, data were collected concerning any family history of diabetes and hypertension, age, obstetric history, parity, height, previous and current weight, the BMI, SBP and DBP, gestational age, and laboratory parameters. At the end of pregnancy, data were collected on gestational complications, the development of PIH and its clinical manifestations (gestational hypertension or preeclampsia), the type of treatment (diet or insulin), the type of delivery (eutocic, dystocic, cesarean section), weeks of termination of pregnancy, newborn infant weight, the Apgar test, and complications in the newborn infant (hypoglycemia, hyperbilirubinemia, infections, and admissions to the neonatal Intensive Care Unit [ICU]).
Following Marín et al.,4 gestational arterial hypertension (AHT) was defined as hypertension diagnosed after week 20 of pregnancy, with no presence of proteinuria, including at the time of delivery, and with a return to normal BP levels after 12 weeks postpartum. Preeclampsia in turn was defined as hypertension with values over 140/90mmHg in normotensive women after week 20 of pregnancy on at least two occasions spaced four hours apart and accompanied by proteinuria >0.3g/24h (or protein/creatinine ratio ≥300mg/g).
A total of 123 pregnant women with normal BP diagnosed with GDM were included in the study. Between weeks 28 and 32 of pregnancy, ABPM was performed at 08:30–09:30h (Spacelabs 90207), with the monitor being programmed to perform readings every 20min during the daytime period and every 30min during the night. We finally excluded 30 patients who did not meet the requirements for accepting the ABPM readings as valid (i.e., at least 66% of theoretical readings made, with at least one reading per hour, and a minimum of 14 readings in the daytime period and 7 readings during the night). A total of 94 pregnant women were therefore included in the study. The following ABPM circadian patterns were established: dipper pattern (BP decrease >10% in the nocturnal period as compared to the daytime period); extreme dipper (BP decrease >20% in the nocturnal period as compared to the daytime period), non-dipper (BP decrease <10% in the nocturnal period as compared to the daytime period); and riser pattern (increased BP in the resting period as compared to the nocturnal period).
The study was approved by the Research Ethics Committee of Hospital Universitario Puerta del Mar. The international ethical recommendations of the Declaration of Helsinki were followed.
Statistical analysisThe descriptive analysis of qualitative variables was based on the calculation of frequencies and percentages, while quantitative variables were reported as the mean and standard deviation (SD) for variables with a normal distribution, and as the median and range for those with a non-normal distribution. After confirming normal data distribution in the sample using the Kolmogorov–Smirnov test, quantitative variables between independent groups were compared using the Student t-test or Mann–Whitney U-test for nonparametric contrasting, while the chi-squared test (or Fisher's exact test, as required) was used to compare qualitative variables between independent groups. All significance values refer to two-tailed testing. Statistical significance was considered for p<0.05.
For each BP modality we plotted the receiver operating characteristic (ROC) curves in order to determine the cut-off points of each of them that best predicted the development of PIH.
Finally, a multivariate analysis was performed using binary logistic regression models. The independent variables to be included in the models were selected based on clinical and statistical criteria (p<0.05 in bivariate analysis), establishing the criterion for inclusion in the model as 0.10 and the criterion for exclusion as 0.15. We performed manual entry into the models of those variables, yielding a significance of p<0.30 in the bivariate analysis. Likewise, we determined the need to retain in the model those nonsignificant variables that induced relevant changes in the coefficients of the rest of the model variables upon being suppressed. Goodness of fit of the final model was assessed using the Hosmer–Lemeshow test. The data were coded, entered and analyzed using the SPSS version 15.0 statistical package for MS Windows.
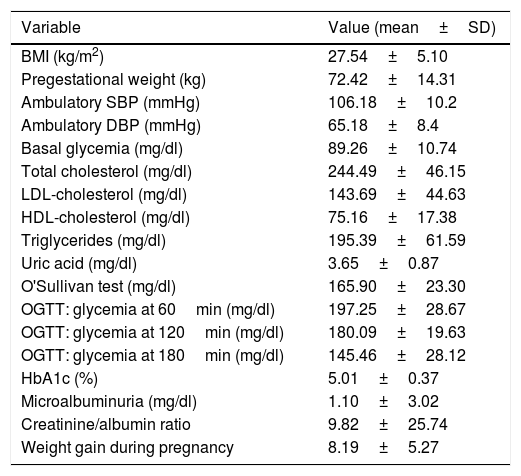
ResultsThe pregnant women included in the analysis (n=93) had a mean age of 34.8±4.39 years. A history of diabetes (types 1 and 2) in a first-degree relative was reported by 43 of the patients (46.2%), and 34 had a history of arterial hypertension in a first-degree relative (36.6%). Twenty-six of the patients had a history of GDM in previous pregnancies (28%). Table 1 describes the clinical and laboratory test data of the patients.
Laboratory test data and clinical variables of the study population.
| Variable | Value (mean±SD) |
|---|---|
| BMI (kg/m2) | 27.54±5.10 |
| Pregestational weight (kg) | 72.42±14.31 |
| Ambulatory SBP (mmHg) | 106.18±10.2 |
| Ambulatory DBP (mmHg) | 65.18±8.4 |
| Basal glycemia (mg/dl) | 89.26±10.74 |
| Total cholesterol (mg/dl) | 244.49±46.15 |
| LDL-cholesterol (mg/dl) | 143.69±44.63 |
| HDL-cholesterol (mg/dl) | 75.16±17.38 |
| Triglycerides (mg/dl) | 195.39±61.59 |
| Uric acid (mg/dl) | 3.65±0.87 |
| O'Sullivan test (mg/dl) | 165.90±23.30 |
| OGTT: glycemia at 60min (mg/dl) | 197.25±28.67 |
| OGTT: glycemia at 120min (mg/dl) | 180.09±19.63 |
| OGTT: glycemia at 180min (mg/dl) | 145.46±28.12 |
| HbA1c (%) | 5.01±0.37 |
| Microalbuminuria (mg/dl) | 1.10±3.02 |
| Creatinine/albumin ratio | 9.82±25.74 |
| Weight gain during pregnancy | 8.19±5.27 |
SD: standard deviation; DBP: diastolic blood pressure; SBP: systolic blood pressure; OGTT: oral glucose tolerance test.
The mean BP values recorded in 24-h ABPM were as follows: mean 24-h SBP: 107.06±9.40mmHg; mean SBP in activity period: 109.23±9.73mmHg; mean SBP in resting period: 102.61±10.28mmHg; mean 24-h DBP: 65.77±6.47mmHg; mean DBP in activity period: 68.01±6.65mmHg; mean DBP in resting period: 61.09±7.50mmHg.
With regard to the obstetric outcomes, 49 of the patients were treated with insulin (52.7%); 45 had eutocic delivery (48.9%); 24 required instrumental delivery (26.1%); and 23 required cesarean section (25%).
With regard to the perinatal outcomes, 6 infants presented delayed intrauterine growth (
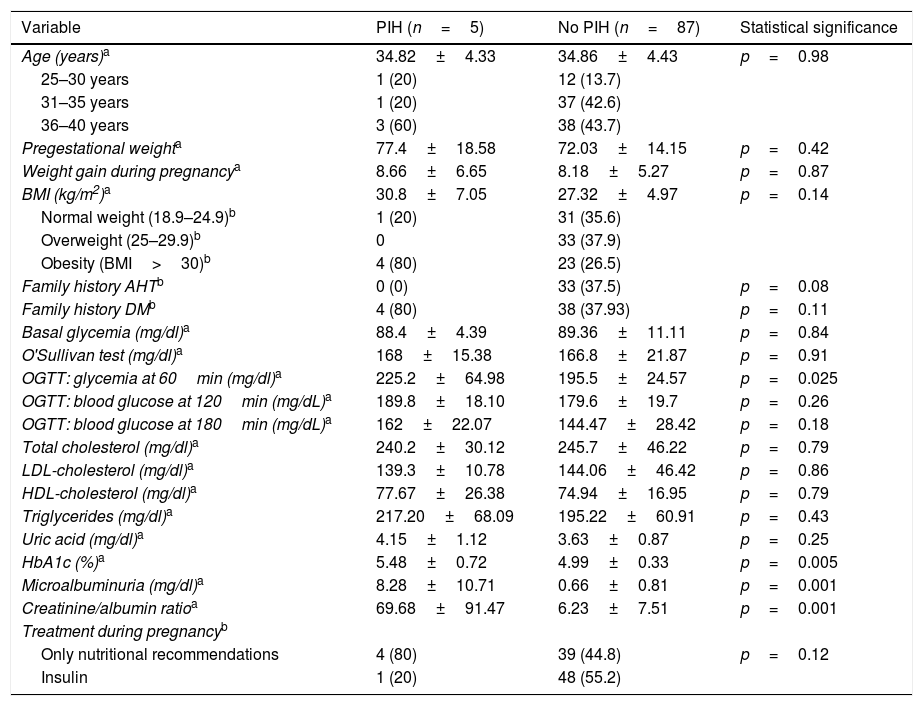
In terms of the maternal outcomes, 5 women developed gestational arterial hypertension (two in week 32, one in week 36, one in week 39, and one in the immediate postpartum period) (5.4%), while one developed preeclampsia in the immediate postpartum period (1.1%). In total, 5 developed some type of hypertensive disorder of pregnancy (gestational arterial hypertension and/or preeclampsia) (5.4%). Table 2 compares laboratory test, demographic and clinical parameters between the patients who developed PIH and those who did not.
Bivariate analysis of the association between the presence of PIH and demographic, laboratory test and clinical parameters.
| Variable | PIH (n=5) | No PIH (n=87) | Statistical significance |
|---|---|---|---|
| Age (years)a | 34.82±4.33 | 34.86±4.43 | p=0.98 |
| 25–30 years | 1 (20) | 12 (13.7) | |
| 31–35 years | 1 (20) | 37 (42.6) | |
| 36–40 years | 3 (60) | 38 (43.7) | |
| Pregestational weighta | 77.4±18.58 | 72.03±14.15 | p=0.42 |
| Weight gain during pregnancya | 8.66±6.65 | 8.18±5.27 | p=0.87 |
| BMI (kg/m2)a | 30.8±7.05 | 27.32±4.97 | p=0.14 |
| Normal weight (18.9–24.9)b | 1 (20) | 31 (35.6) | |
| Overweight (25–29.9)b | 0 | 33 (37.9) | |
| Obesity (BMI>30)b | 4 (80) | 23 (26.5) | |
| Family history AHTb | 0 (0) | 33 (37.5) | p=0.08 |
| Family history DMb | 4 (80) | 38 (37.93) | p=0.11 |
| Basal glycemia (mg/dl)a | 88.4±4.39 | 89.36±11.11 | p=0.84 |
| O'Sullivan test (mg/dl)a | 168±15.38 | 166.8±21.87 | p=0.91 |
| OGTT: glycemia at 60min (mg/dl)a | 225.2±64.98 | 195.5±24.57 | p=0.025 |
| OGTT: blood glucose at 120min (mg/dL)a | 189.8±18.10 | 179.6±19.7 | p=0.26 |
| OGTT: blood glucose at 180min (mg/dL)a | 162±22.07 | 144.47±28.42 | p=0.18 |
| Total cholesterol (mg/dl)a | 240.2±30.12 | 245.7±46.22 | p=0.79 |
| LDL-cholesterol (mg/dl)a | 139.3±10.78 | 144.06±46.42 | p=0.86 |
| HDL-cholesterol (mg/dl)a | 77.67±26.38 | 74.94±16.95 | p=0.79 |
| Triglycerides (mg/dl)a | 217.20±68.09 | 195.22±60.91 | p=0.43 |
| Uric acid (mg/dl)a | 4.15±1.12 | 3.63±0.87 | p=0.25 |
| HbA1c (%)a | 5.48±0.72 | 4.99±0.33 | p=0.005 |
| Microalbuminuria (mg/dl)a | 8.28±10.71 | 0.66±0.81 | p=0.001 |
| Creatinine/albumin ratioa | 69.68±91.47 | 6.23±7.51 | p=0.001 |
| Treatment during pregnancyb | |||
| Only nutritional recommendations | 4 (80) | 39 (44.8) | p=0.12 |
| Insulin | 1 (20) | 48 (55.2) |
No statistically significant differences were found on analyzing the association between the presence of PIH and the obstetric and perinatal outcomes (macrosomia, small for gestational age, delayed intrauterine growth, hyperbilirubinemia, hypoglycemia, congenital malformations).
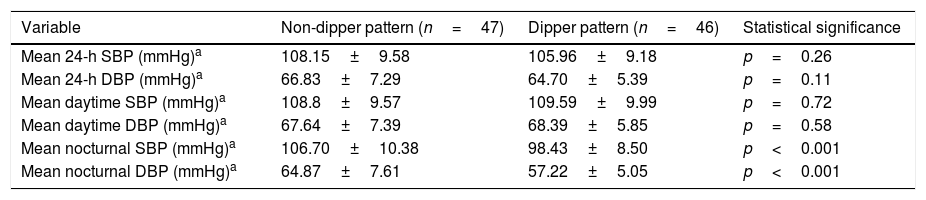
With regard to ABPM, 47 patients had a non-dipper pattern (50.5%) and 46 had a dipper pattern (49.5%). The nocturnal averages were significantly higher in patients with a non-dipper pattern than in those with a dipper pattern. No significant differences were found between the two groups in relation to the daytime and 24-h blood pressure parameters or the other clinical and laboratory test parameters (Table 3).
Bivariate analysis of the association between the presence of dipper/non-dipper pattern evidenced by ABPM and the blood pressure parameters analyzed.
| Variable | Non-dipper pattern (n=47) | Dipper pattern (n=46) | Statistical significance |
|---|---|---|---|
| Mean 24-h SBP (mmHg)a | 108.15±9.58 | 105.96±9.18 | p=0.26 |
| Mean 24-h DBP (mmHg)a | 66.83±7.29 | 64.70±5.39 | p=0.11 |
| Mean daytime SBP (mmHg)a | 108.8±9.57 | 109.59±9.99 | p=0.72 |
| Mean daytime DBP (mmHg)a | 67.64±7.39 | 68.39±5.85 | p=0.58 |
| Mean nocturnal SBP (mmHg)a | 106.70±10.38 | 98.43±8.50 | p<0.001 |
| Mean nocturnal DBP (mmHg)a | 64.87±7.61 | 57.22±5.05 | p<0.001 |
On analyzing the relationship between the circadian pattern and the different perinatal obstetric complications, we found patients with a non-dipper pattern in ABPM to present fewer weeks of gestation at delivery as compared to those with a dipper pattern (38.67±1.60 vs. 39.27±1.11, respectively; p=0.04). We also found that newborn infants of mothers with a non-dipper pattern had lower birth weight than newborn infants of mothers with normal ABPM findings (3084.57±515.2 vs. 3323.7±457.9, respectively; p=0.021). No statistically significant differences were found in relation to the rest of the obstetric or perinatal results.
We likewise observed no differences between patients with a non-dipper pattern and the development of PIH, cesarean section, the need for newborn admission to the ICU, or macrosomia.
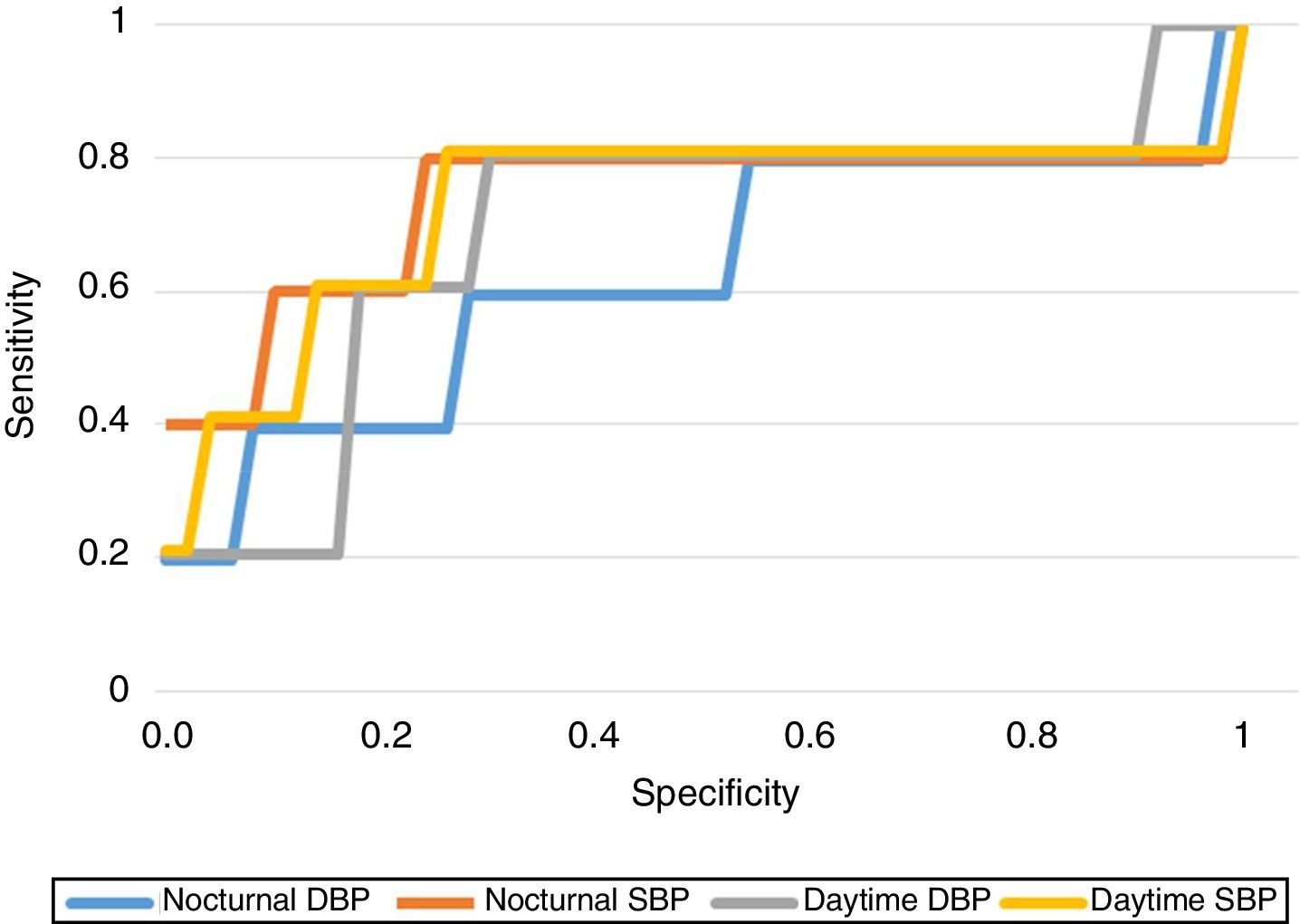
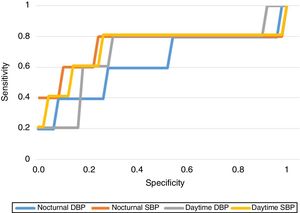
As regards the predictive capacity of the BP results obtained, we established the BP cut-off points offering the greatest sensitivity (Se) and specificity (Sp) in predicting the development of PIH, obtaining a daytime SBP of 113.5mmHg (Se 80%, Sp 77.6%), a daytime DBP of 70.5mmHg (Se 80%, Sp 69%), a nocturnal SBP of 108.5mmHg (Se 80%, Sp 77%) and a nocturnal DBP of 58.5mmHg (Se 80%, Sp 43.7%). Fig. 1 shows the ROC curves of each of the BP ranges.
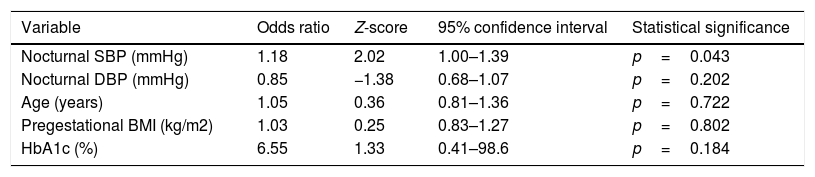
The logistic regression analysis performed to explore the association between ABPM (nocturnal SBP and DBP) and the development of PIH, including some laboratory test and demographic parameters (age, the BMI and HbA1c), confirmed that nocturnal SBP presented a significant odds ratio (OR) of 1.18 (95%CI: 1.00–1.39; p=0.043), as seen in Table 4. This OR finding is interpreted in a way similar to adjusted relative risk, i.e., on average, an increase of 1mmHg in nocturnal SBP can increase the probability of experiencing PIH according to this model by 18%, assuming all other variables are stable. The Hosmer–Lemeshow statistic (9.18 for 6 degrees of freedom (df); p=0.16) indicates an adequate fit to the logistic regression model.
Logistic regression analysis of pregnancy-induced hypertension (n=94).
| Variable | Odds ratio | Z-score | 95% confidence interval | Statistical significance |
|---|---|---|---|---|
| Nocturnal SBP (mmHg) | 1.18 | 2.02 | 1.00–1.39 | p=0.043 |
| Nocturnal DBP (mmHg) | 0.85 | −1.38 | 0.68–1.07 | p=0.202 |
| Age (years) | 1.05 | 0.36 | 0.81–1.36 | p=0.722 |
| Pregestational BMI (kg/m2) | 1.03 | 0.25 | 0.83–1.27 | p=0.802 |
| HbA1c (%) | 6.55 | 1.33 | 0.41–98.6 | p=0.184 |
DBP: diastolic blood pressure; SBP: systolic blood pressure.
This study shows that patients with GDM present subclinical blood pressure changes detected by ABPM, with a predominance of a non-dipper pattern and high nocturnal SBP and DBP values, and we believe that these findings could be predictive of PIH.
As described in the literature, age is an important and non-modifiable risk factor for the development of both GDM12 and PIH.4 In our study, 60% of the patients with PIH were 36–40 years of age; no significant difference could be shown, probably due to the limited sample size involved.
Obesity is the most important modifiable risk factor for the development of GDM13 and PIH.4,14 Our study was also consistent with this, for although the most common BMI values were in the overweight range, the BMI most frequently (80%) associated with PIH corresponded to the obesity range (BMI >30kg/m2). Patients who developed PIH had a greater BMI, greater pregestational weight, and greater weight gain at the end of pregnancy.
A number of studies indicate that a family history of arterial hypertension and DM increases the risk of blood pressure changes during pregnancy.4,14 However, only 33 of the patients in our series (37.5%) reported a history of arterial hypertension, and none of them developed PIH. In contrast, four of the 5 patients who developed PIH had a family history of DM.
As regards glycemic control, and in coincidence with other publications,15 we found HbA1c concentration to significantly influence the development of PIH. With regard to the potential effects of insulin therapy, four patients who developed PIH (80%) were receiving dietary treatment, while one received insulin therapy (20%). These differences were not significant.
Different studies have suggested that microalbuminuria could be regarded as a predictor of preeclampsia in early pregnancy.16 Its presence has been correlated to factors associated with insulin resistance and endothelial dysfunction in patients with type 1 DM,17 type 2 DM18 and GDM.19 These findings agree with those of our own study, where microalbuminuria was seen to be higher in patients who developed PIH. In turn, the albumin/creatinine ratio has also been regarded as a predictor of preeclampsia in early gestational stages among pregnant women with20 and without GDM.21
We only found PIH in 5 of the patients with GDM (5.4%), this figure being lower than the incidence reported in the literature.22 This lower incidence may be due to the fact that our study focused on late-onset GDM (the patients were recruited in the third trimester of pregnancy: 28–32 weeks). The percentage of women with larvated type 2 DM diagnosed in pregnancy as GDM therefore represents a very small percentage. This precludes the possibility of recruiting pregnant women with pregestational DM who are included as gestational diabetes in many studies and therefore increase the incidence of PIH and of future cardiovascular complications. In fact, in the study published by Sullivan et al.,22 the prevalence of preeclampsia was reported to be 5–8% of all pregnancies, with the figure increasing to 20% in patients with pregestational diabetes. The prevalence in GDM proved lower and variable (<20%). However, identifying potential predictors of increased risk in pregnant women with late-onset GDM would be very useful for ascertaining the true impact upon perinatal morbidity and future cardiovascular risk.
The incidence of PIH in patients with a non-dipper circadian pattern was lower than expected, because the literature describes this pattern as being more commonly associated with both PIH and preeclampsia, regardless of the mean BP value.23,24 In our study, the non-dipper pattern was more prevalent than the dipper pattern, which is consistent with the data obtained both in patients with GDM25 and in patients with type 1 DM26 and type 2 DM.27
It should be noted from our results that patients with a non-dipper pattern had significantly higher SBP and DBP levels during the nocturnal hours. Despite this, however, on performing the multivariate analysis, only nocturnal SBP was identified as an independent predictor. Similarly, patients with high mean SBP and DBP values (>120 and >70mmHg, respectively) typically exhibited a non-dipper pattern.
Our results have allowed us to establish BP cut-off points (108.5mmHg) of high specificity and sensitivity in predicting PIH; this largely coincides with the data published in relation to pregnant women with type 1 DM.11
Of the newborn infants, 24 had low birth weight (26.1%) and 16 macrosomia (17.4%). Although the association of these findings with GDM is well known,28 the potential role which subclinical BP changes could play in them is uncertain, particularly in relation to low birth weight.29
Twenty-three of the patients (25%) required cesarean section, this figure being somewhat lower than that reported in the literature.30 However, in our study we were unable to establish a significant relationship between the indication of cesarean section and the presence of PIH, preeclampsia, or altered ABPM patterns.
The disadvantages of our study include the fact that the reproducibility of ABPM is limited, since values that underestimate or overestimate the detected blood pressure alterations may be obtained. Moreover, there are no normality reference values against which our findings may be contrasted, and the incidence of PIH in the studied population was lower than expected. However, we have observed trends that could have great clinical significance, although such findings need to be supported by future studies involving larger samples (even though ours is the largest sample size published to date). In addition, few studies have been published on blood pressure changes detected by ABPM in women with GDM who develop PIH. However, we consider this to be a strength, due to the importance of our having identified potential predictors of the development of PIH in pregnant women with GDM, which could help to ascertain its impact on perinatal morbidity and the future risk of type 2 DM and cardiovascular disease. This in turn could be of great value to the healthcare system.
ConclusionsSubclinical blood pressure changes can be seen in GDM, fundamentally involving a predominance of non-dipper patterns and higher nocturnal SBP and DBP values, which in turn may be predictive of PIH. Studies with larger sample sizes are required to confirm these findings and to establish the relationship between blood pressure changes and maternal and perinatal complications.
AuthorshipCLT and MAD designed the study. BSL and ALB conducted the fieldwork and entered the data for analysis. AM and AA performed blood sampling and applied ABPM. BSL collected the obstetric data. JACD participated in the design of the data analytical strategy. CLT, MAD and BSL prepared the first manuscript draft and incorporated suggestions from all the co-authors. All the authors have read and approved the final version of this article.
Financial supportSpanish Society of Endocrinology and Nutrition Foundation research award for young endocrinologists 2016.
2013–2016 State Plan, ISCIII, reference code: PI 2016 G72109168, and FEDER.
Instituto de Investigación Biomédica e Innovación de Cádiz (INIBICA).
Conflicts of interestThe authors of this article have no conflicts of interests in relation to the objective or the results of this article.
Please cite this article as: Sánchez-Lechuga B, Lara-Barea A, Córdoba-Doña JA, Montero Galván A, Abal Cruz A, Aguilar-Diosdado M, et al. Utilidad de la monitorización ambulatoria de la presión arterial en mujeres con diabetes mellitus gestacional. Endocrinol Diabetes Nutr. 2018;65:394–401.