The purpose of this study is to identify the role of diabetes mellitus in the effectiveness of intradialytic exercise intervention among haemodialysis patients.
MethodsIn this multicentre study 90 dialysed patients were allocated to the experimental (EXG, n=57) or control group (CNG, n=33). In EXG, we included 20 diabetic and 37 non-diabetic patients. In CNG, we enrolled 8 diabetic and 25 non-diabetic patients. EXG underwent a 12-week supervised, progressive, intradialytic resistance training programme, while CNG stayed inactive during dialysis. Baseline, post-interventional and post-follow-up assessments of maximal force during hip extension (HE), hip flexion (HF) and knee extension (KE) contractions were completed in both groups of patients.
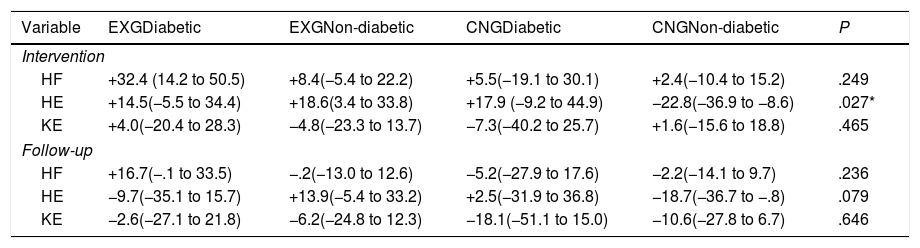
ResultsHE increased in diabetic and non-diabetic EXG patients (diabetic EXG, change: +14.5N; 95% CI=−5.5 to +34.5; non-diabetic EXG, +18.6N; 95% CI=+3.4 to +33.8) and diabetic CNG patients (change: +17.9N; 95% CI=−9.2 to +44.9). Only non-diabetic CNG patients experienced a decrease in HE (change: −22.8N; 95% CI=−36.9 to −8.7, P<.05).
ConclusionsResistance training improved muscle function among dialysis patients regardless of the presence of diabetes mellitus. We found that non-diabetic patients lose their muscle function extensively during inactivity, while diabetic patients retain their muscle function.
El objetivo de este estudio era identificar el papel de la diabetes mellitus en la eficacia de la intervención con ejercicio intradialítico en pacientes en hemodiálisis.
MétodosEn este estudio multicéntrico se asignó a 90 pacientes dializados al grupo experimental (GEX, n=57) o al grupo de control (GC, n=33). Se incluyó en el GEX a 20 pacientes diabéticos y 37 no diabéticos. En el GC se incluyó a ocho pacientes diabéticos y a 25 no diabéticos. El GEX se sometió a un programa de entrenamiento de resistencia intradialítico progresivo supervisado durante 12 semanas, mientras que el GC permaneció inactivo durante la diálisis. Se hicieron en los dos grupos de pacientes valoraciones basales, tras la intervención y después del seguimiento de la fuerza máxima durante contracciones de extensión de la cadera (EC), flexión de la cadera (FC) y extensión de la rodilla (ER).
ResultadosLa EC aumentó en los pacientes diabéticos y no diabéticos del GEX (GEX diabético, cambio: +14,5N; IC 95%=−5,5 a +34,5; GEX no diabético, +18,6N; IC 95%=+3,4 a +33,8) y en los pacientes del GC diabéticos (cambio: +17,9N; IC 95%=−9,2 a +44,9). Solo los pacientes del GC no diabéticos mostraron un descenso de la EC (cambio: −22,8N; IC 95%=−36,9 a −8,7, p<0,05).
ConclusionesEl entrenamiento de resistencia mejoró la función muscular en los pacientes en diálisis, con independencia de la presencia de diabetes mellitus. Hallamos en los pacientes no diabéticos una pérdida acusada de la función muscular durante la inactividad, mientras que los diabéticos conservan la función muscular.
Among haemodialysis (CKD-5D) patients, diabetic nephropathy is one of the most frequent diagnoses existing in parallel with diabetes mellitus and is associated with negative effects on a patients’ physical abilities, muscle mass and muscle function.1–4
The gradual loss of skeletal muscle mass and function accelerates especially after the initiation of intermittent haemodialysis sessions. Muscle protein breakdown increases due to changes in endocrine system function, while muscle protein synthesis decreases due to inappropriate protein intake, extraction of amino acids during dialysis therapy and the prevalence of “anabolic resistance” among CKD-5D patients.5,6 These disturbances in the balance of muscle protein breakdown and synthesis accelerate catabolic processes and deteriorations in muscle mass and function. A low body mass index with the presence of diabetes and the gradual loss of muscle mass and function have been identified as independent mortality predictors in CKD-5D patients.7,8
To minimize diabetes and kidney disease-related health issues effectively, lifestyle management, including physical activity counselling, is strongly recommended.9 The Renal Association Clinical Practice Guideline recommends that all dialysed patients without contraindication should perform at least 30min of supervised moderate intensity exercise during a dialysis session.10 Exercise interventions containing resistance training showed high efficiency in maintaining patients’ muscle mass,11 and muscle function,12,13 reduced the prevalence of frailty,14 improved arterial elasticity,15 mitochondrial function,16 substrate oxidation processes,15,17 insulin sensitivity,11 insulin signalling17 and glycaemic control18 in the healthy population as well as in patients with diabetes. However, in the literature we found greater individual differences in physiological and functional adaptation to resistance training among dialysed patients compared to healthy and diabetic subjects. For a better understanding of the parameters that affect adaptation to resistance training among CKD-5D patients, we assessed the interactions between the presence of diabetes mellitus and the change in muscle function after an intradialytic resistance training programme.
Material and methodsStudy designWe conducted this two-group, pre-post comparative study in 2018 at three dialysis centres (Fresenius Medical Care Dialysis Services in Kosice, Logman East in Kosice and Fresenius Medical Care Dialysis Services in Banska Bystrica). The study design and protocol were approved by the Ethics Committee of Pavol Jozef Safarik University in Kosice (approval no. 14N/2017) and all methods, assessments and data acquisitions were conducted in accordance with the Declaration of Helsinki of 1975 and with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. Written informed consent was obtained from all study subjects. The official protocol of the study was registered at ClinicalTrials.gov (ID: NCT03511924) before the onset of patient enrolment.19 The detailed description of study implementation was provided elsewhere.20
Study subjectsAll CKD-5D patients from three dialysis centres (n=198) were screened and selected according to the three inclusion and four exclusion criteria. The inclusion criteria were: 1. patients diagnosed with end-stage renal disease, 2. patients who were over 30 years of age, 3. patients who had been receiving treatment by maintenance haemodialysis therapy for at least the last three months and continued with dialysis therapy during the implementation of the study. The exclusion criteria were: 1. patients who had lower extremity amputation, 2. patients who had severe dementia or retardation, 3. patients who had an acute intercurrent disease, 4. patients with a probability of one-year mortality higher than 25% according to the Charlson Comorbidity Index.21
InterventionPatients attending dialysis therapy at both sites in Kosice were allocated to the experimental group (EXG, n=57), while patients from the Banska Bystrica dialysis centre were allocated to the control group (CNG, n=33). After the allocation procedure, the research team members and participating patients were informed about the group assignment structure.
Experimental conditionsPatients allocated to the EXG group participated in a 12-week intradialytic resistance training (IRT) programme, which they performed under the supervision of training assistants three times per week. The IRT sessions were 40min in length and composed of 3-min of warming-up, 30-min of conditioning and 7-min of cooling-down and stretching. To perform effective exercises in a supine position during dialysis, we used external pressure generated by elastic bands and over-balls (TheraBand®, Akron, OH, USA). These external loading resources were fixed on a construction of the dialysis bed, and during exercises patients pulled or pushed against them. The programme included three exercises (A. a unilateral push and pull of the over-ball against a leg board, B. a bilateral knee squeezing of the over-ball, and C. a unilateral straight leg raise against the band pressure).
The progress of the IRT programme was individual and depended on the physical capabilities of the patient. During the first two weeks of the IRT programme, patients performed three sets (12 up to 15 repetitions each) of three different exercises of lower extremity muscles per session. Once a patient was capable of safely completing the planned programme for the relevant session, the numbers of repetitions in the next session increased by three repetitions for each exercise. If a patient reached the maximal number of repetitions per exercise (18 repetitions) during the session, then for the next session the number of sets was increased by one set, and the initial number of repetitions per exercise became 12. When the patient was able to perform five sets with 18 repetitions for each exercise, we made the IRT harder by applying a stiffer elastic band or an over-ball with higher hardness. In contrast, if a patient failed to complete the entire training session or had obvious difficulties, the IRT was facilitated by lowering the number of repetitions per set, or of sets, or by the use of softer elastic bands and over-balls. This methodology of training progressivity enabled us to maintain the patient's safety during IRT and ensured the subjective intensity of training to be between “moderate” and “hard”. To control the patient's training progress during IRT, we registered the number of repetitions and series for each of exercise independently in the patient's training log-book.
Control conditionPatients allocated to the CNG group received their standard nephrology care. Through the 12-weeks control period, all CNG patients maintained their standard treatment regimen and maintained their customary dietary and physical activity patterns. The CNG patients were informed about the clinical benefits and effects of regular physical activity in dialysed patients, and during the control period they were received increased attention from the research team members.
Follow-up conditionAll patients enrolled in the study underwent a 12 weeks follow-up period after the completion of the experimental or control condition. During the follow-up, the patients were not involved in any organized physical activity during the dialysis and returned to their original HT regimen.19
MeasuresThe primary outcome of the study was the change of maximal isometric force generated during the contractions of the lower extremity muscles involved in hip and knee joint movements. A detailed description of the outcome assessments is described in the protocol article.17 Maximal isometric forces generated during the three lower extremity movements (hip extension, HE; hip flexion, HF; and knee extension, KE) were assessed by a hand-held dynamometer (Universal digital force gauge HF 500, SAUTER GmbH, Balingen, Germany). The assessments of maximal isometric contraction force realized by hand-held dynamometers showed an excellent inter-rater reliability (Hip extension Interclass correlation coefficient – ICC: .92–.95, standard error of measurement – SEM: 5.34–7.29, minimal detectable change – MDC: 10.46–14.29; hip flexion ICC: .92–.93, SEM: 6.39–6.71, MDC: 12.53–13.15; knee extension ICC: .89–.90, SEM: 8.76–9.30, MDC: 17.18–18.23) and concurrent validity (hip extension ICC: .88–.94; hip flexion ICC: .92–.94; knee extension: ICC .82–.92) compared with the results received from the isokinetic dynamometer.22 During the assessments, patients were in a supine position with arms safely and comfortably placed on the bed. During the assessments of the HE and HF of the lower limb the patient held the dominant leg in a straightened position, while the dynamometer was placed proximally to the ankle, on the anterior surface of the lower leg for the HF force assessments and on the posterior surface of the lower leg for the HE force assessments. The KE measurements of the dominant leg were done at a knee angle of 90° from full extension. The hand-held, portable dynamometer was placed on the patient's ankle and was stabilized during the performance of the physical examination. The patients were instructed to perform a maximal isometric contraction and hold it for 5s. The tests were repeated with 30-second rest intervals, and the higher measured values of two consecutive tests were used for the analysis. The changes of maximal isometric forces were calculated as 1. post-intervention measures and 2. post-follow-up measures minus the baseline measure (measure unit: Newton; N).
Background variables regarding a patient's clinical data were extracted from the latest electronic medical record of the patient completed before the start of the intervention. The extracted data contained A. patient's age and gender, B. body composition parameters (body weight; body height; body mass index calculated as body weight in kilograms divided by the square of the body height in metres (BMI, kg/m2), lean tissue mass measured by the body composition monitor23 and lean tissue index calculated as lean tissue mass/height2) and C. nephrological clinical data containing the presence of diabetes mellitus (Yes/No), total length of dialysis (in months), dialysis adequacy (Kt/V), degree of over-hydration (%), and concentrations of C-reactive protein, parathyroid hormone, haemoglobin, albumin, ferritin, phosphates, calcium, potassium and sodium.
The primary outcome measures, regarding a maximal isometric force during lower extremities contractions, were collected in both groups before and after the 12-week experimental and control condition and also after the 12-week follow-up. The primary outcome measures were assessed by the same investigator and were completed in the first hour of the patient's haemodialysis session. The background variables, regarding a patient's age and gender, body composition parameters and nephrological clinical data were collected only before the start of the intervention.
Statistical analysisFirst, we assessed background variables and compared them between the EXG and CNG groups using χ2 tests for categorical (binary) variables and the Student's t-test for continuous variables for possible differences.
Second, we assessed whether the presence of diabetes mellitus interacted with the change in the indices of maximal forces after the intervention and follow-up, using (2×2) two-way ANOVA tests. We performed all data analyses on a complete-case analysis basis, i.e. including only patients with complete baseline, post-intervention and post-follow-up assessments. The level of significance was set at an α level of .05 and data analyses were carried out using the statistical software package IBM SPSS 22.0 (IBM Corp. Released 2013, IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
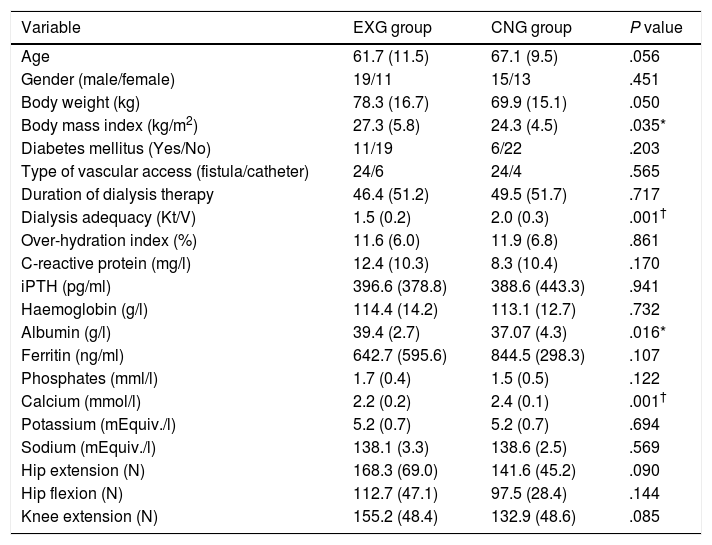
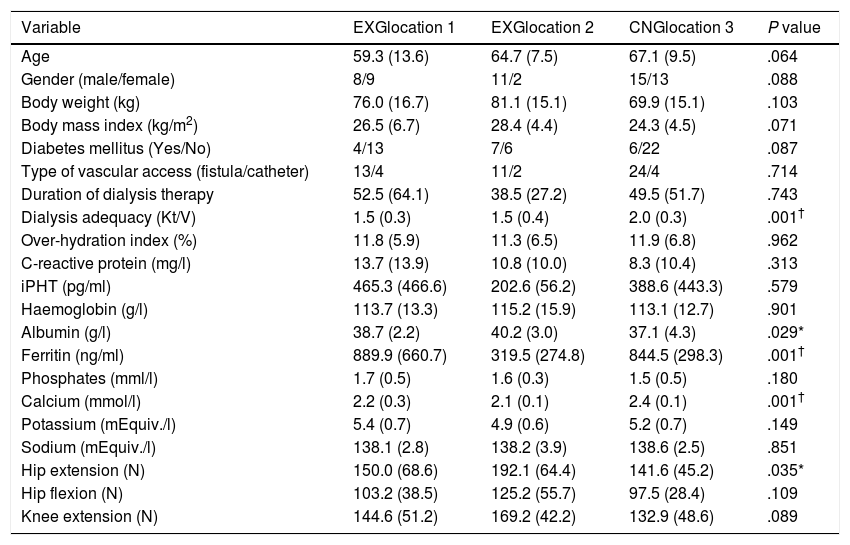
ResultsPatients’ characteristics and flowDuring the recruitment of participants, 198 dialysis patients were screened and selected through their nephrologists according to the inclusion and exclusion criteria, yielding 126 eligible patients (63.6% of eligible patients). These received oral and written information about the possibility of participating in the study, leading to 90 patients signing a written informed consent (71.4% response rate) prior to the study. Patients attending dialysis therapy at both sites in Kosice were allocated to the experimental group (EXG, n=57), while patients from the Banska Bystrica dialysis centre were allocated to the control group (CNG, n=33). After the allocation procedure, the investigatory team members and participating patients were informed about the group assignment structure. Baseline patient characteristics and their differences between EXG and CNG are summarized in Table 1. Baseline characteristics of patients according to the location of dialysis centres where they were receiving dialysis therapy are summarized in Table 2. In the baseline comparison, we found significant differences in body mass index, dialysis adequacy and concentrations of albumin, ferritin and calcium between the groups. No significant differences were found in the presence of diabetes mellitus and type of vascular access used for haemodialysis therapy between the study groups. We found that for dialysis vascular access, an arteriovenous fistula (83%) was used more often compared to a central venous catheter (17%). All patients were treated with online hemodiafiltration using polysulphone dialysers. Smartbags of dialysis concentrates were used as a dialysis bath in all three centres. Dialysers and bath choice was not dependent on participation in the study and was fully under the responsibility of medical directors of the dialysis centre who adapt the patient's treatment according to the clinical results. All three centres treat their patients according the same general guidelines. Dialysis centres that participated in the study providing haemodialysis care according to the national standards for quality of dialysis care. Participation in the study was not a driver of any changes in prescription.
Baseline patient characteristics and their differences between the EXG and CNG groups.
| Variable | EXG group | CNG group | P value |
|---|---|---|---|
| Age | 61.7 (11.5) | 67.1 (9.5) | .056 |
| Gender (male/female) | 19/11 | 15/13 | .451 |
| Body weight (kg) | 78.3 (16.7) | 69.9 (15.1) | .050 |
| Body mass index (kg/m2) | 27.3 (5.8) | 24.3 (4.5) | .035* |
| Diabetes mellitus (Yes/No) | 11/19 | 6/22 | .203 |
| Type of vascular access (fistula/catheter) | 24/6 | 24/4 | .565 |
| Duration of dialysis therapy | 46.4 (51.2) | 49.5 (51.7) | .717 |
| Dialysis adequacy (Kt/V) | 1.5 (0.2) | 2.0 (0.3) | .001† |
| Over-hydration index (%) | 11.6 (6.0) | 11.9 (6.8) | .861 |
| C-reactive protein (mg/l) | 12.4 (10.3) | 8.3 (10.4) | .170 |
| iPTH (pg/ml) | 396.6 (378.8) | 388.6 (443.3) | .941 |
| Haemoglobin (g/l) | 114.4 (14.2) | 113.1 (12.7) | .732 |
| Albumin (g/l) | 39.4 (2.7) | 37.07 (4.3) | .016* |
| Ferritin (ng/ml) | 642.7 (595.6) | 844.5 (298.3) | .107 |
| Phosphates (mml/l) | 1.7 (0.4) | 1.5 (0.5) | .122 |
| Calcium (mmol/l) | 2.2 (0.2) | 2.4 (0.1) | .001† |
| Potassium (mEquiv./l) | 5.2 (0.7) | 5.2 (0.7) | .694 |
| Sodium (mEquiv./l) | 138.1 (3.3) | 138.6 (2.5) | .569 |
| Hip extension (N) | 168.3 (69.0) | 141.6 (45.2) | .090 |
| Hip flexion (N) | 112.7 (47.1) | 97.5 (28.4) | .144 |
| Knee extension (N) | 155.2 (48.4) | 132.9 (48.6) | .085 |
Data are presented as mean±standard deviation. iPTH, intact parathyroid hormone; EXG, experimental group; CNG, control group. Differences between groups significant at P<0.05 are denoted by *. Differences between groups significant at P<0.01 are denoted by †.
Baseline patient characteristics and their differences between the patients groups from three dialysis locations.
| Variable | EXGlocation 1 | EXGlocation 2 | CNGlocation 3 | P value |
|---|---|---|---|---|
| Age | 59.3 (13.6) | 64.7 (7.5) | 67.1 (9.5) | .064 |
| Gender (male/female) | 8/9 | 11/2 | 15/13 | .088 |
| Body weight (kg) | 76.0 (16.7) | 81.1 (15.1) | 69.9 (15.1) | .103 |
| Body mass index (kg/m2) | 26.5 (6.7) | 28.4 (4.4) | 24.3 (4.5) | .071 |
| Diabetes mellitus (Yes/No) | 4/13 | 7/6 | 6/22 | .087 |
| Type of vascular access (fistula/catheter) | 13/4 | 11/2 | 24/4 | .714 |
| Duration of dialysis therapy | 52.5 (64.1) | 38.5 (27.2) | 49.5 (51.7) | .743 |
| Dialysis adequacy (Kt/V) | 1.5 (0.3) | 1.5 (0.4) | 2.0 (0.3) | .001† |
| Over-hydration index (%) | 11.8 (5.9) | 11.3 (6.5) | 11.9 (6.8) | .962 |
| C-reactive protein (mg/l) | 13.7 (13.9) | 10.8 (10.0) | 8.3 (10.4) | .313 |
| iPHT (pg/ml) | 465.3 (466.6) | 202.6 (56.2) | 388.6 (443.3) | .579 |
| Haemoglobin (g/l) | 113.7 (13.3) | 115.2 (15.9) | 113.1 (12.7) | .901 |
| Albumin (g/l) | 38.7 (2.2) | 40.2 (3.0) | 37.1 (4.3) | .029* |
| Ferritin (ng/ml) | 889.9 (660.7) | 319.5 (274.8) | 844.5 (298.3) | .001† |
| Phosphates (mml/l) | 1.7 (0.5) | 1.6 (0.3) | 1.5 (0.5) | .180 |
| Calcium (mmol/l) | 2.2 (0.3) | 2.1 (0.1) | 2.4 (0.1) | .001† |
| Potassium (mEquiv./l) | 5.4 (0.7) | 4.9 (0.6) | 5.2 (0.7) | .149 |
| Sodium (mEquiv./l) | 138.1 (2.8) | 138.2 (3.9) | 138.6 (2.5) | .851 |
| Hip extension (N) | 150.0 (68.6) | 192.1 (64.4) | 141.6 (45.2) | .035* |
| Hip flexion (N) | 103.2 (38.5) | 125.2 (55.7) | 97.5 (28.4) | .109 |
| Knee extension (N) | 144.6 (51.2) | 169.2 (42.2) | 132.9 (48.6) | .089 |
Data are presented as mean±standard deviation. EXG location 1, patients located at the Fresenius Kosice; EXG location 2, patients located at the Logman Kosice; CNG location 3, patients located at the Fresenius Banska Bystrica; iPTH, intact parathyroid hormone; EXG, experimental group; CNG, control group. Differences between groups significant at P<0.05 are denoted by *. Differences between groups significant at P<0.01 are denoted by †.
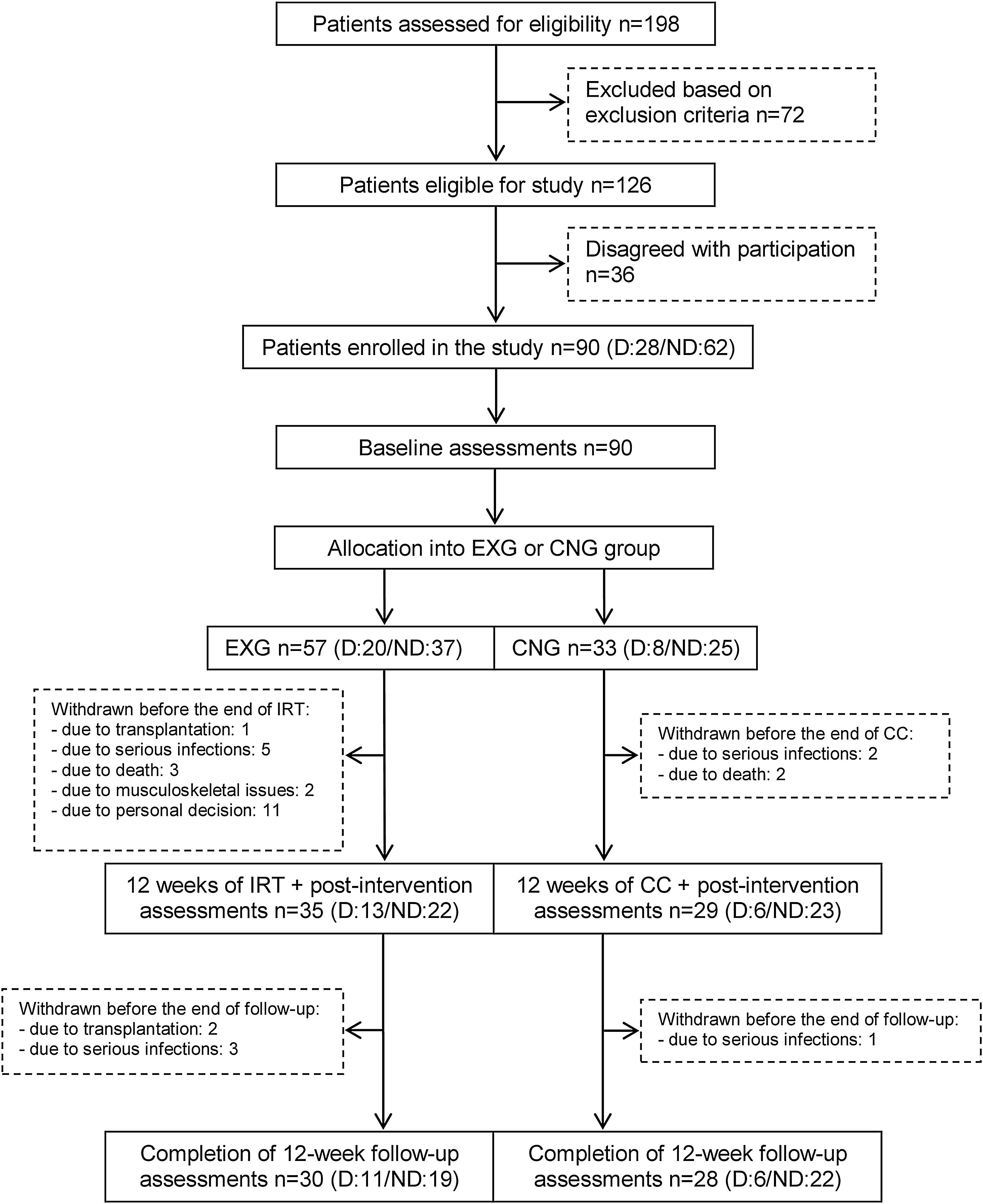
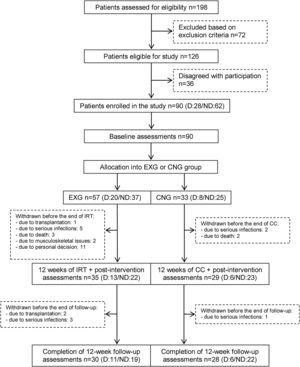
From the initial 90 enrolled patients, 35 EXG patients and 29 CNG patients completed the intervention. In patients that completed the exercise intervention, compliance was adequate, with an average rate of 83%. The post-follow-up assessments were completed in 30 EXG and 28 CNG patients. The final study compliance rate was 64%. A detailed flow summary (Fig. 1) containing the numbers of the patients who completed the intervention and follow-up is presented in the CONSORT diagram.24
CONSORT flow chart of patients summarizing their eligibility assessment, enrolment and allocation to the experimental and control groups of the study, and the drop-out of patients at the two further measurements (EXG: experimental group; CNG: control group; IRT: intradialytic resistance training; CC: control condition).
After intervention, patients with the presence of diabetes mellitus in EXG (change: +14.5N; 95% CI=−5.5 to +34.5) and CNG (change: +17.9N; 95% CI=−9.2 to +44.9) and non-diabetic EXG patients (non-diabetic CNG, change: +18.6N; 95% CI=+3.4 to +33.8) all showed an increase in maximal force during HE isometric contraction. Only CNG patients without the presence of diabetes experienced a decrease in HE (change: −22.8N; 95% CI=−36.9 to −8.7; P<0.05). No significant differences in the changes between groups were found for the other two indices (HF and KE) of lower extremity muscle function (Table 3).
Comparison of changes in muscle function between the experimental (EXG) and control (CNG) group patients regarding the presence of diabetes mellitus after intervention and follow-up.
| Variable | EXGDiabetic | EXGNon-diabetic | CNGDiabetic | CNGNon-diabetic | P |
|---|---|---|---|---|---|
| Intervention | |||||
| HF | +32.4 (14.2 to 50.5) | +8.4(−5.4 to 22.2) | +5.5(−19.1 to 30.1) | +2.4(−10.4 to 15.2) | .249 |
| HE | +14.5(−5.5 to 34.4) | +18.6(3.4 to 33.8) | +17.9 (−9.2 to 44.9) | −22.8(−36.9 to −8.6) | .027* |
| KE | +4.0(−20.4 to 28.3) | −4.8(−23.3 to 13.7) | −7.3(−40.2 to 25.7) | +1.6(−15.6 to 18.8) | .465 |
| Follow-up | |||||
| HF | +16.7(−.1 to 33.5) | −.2(−13.0 to 12.6) | −5.2(−27.9 to 17.6) | −2.2(−14.1 to 9.7) | .236 |
| HE | −9.7(−35.1 to 15.7) | +13.9(−5.4 to 33.2) | +2.5(−31.9 to 36.8) | −18.7(−36.7 to −.8) | .079 |
| KE | −2.6(−27.1 to 21.8) | −6.2(−24.8 to 12.3) | −18.1(−51.1 to 15.0) | −10.6(−27.8 to 6.7) | .646 |
Data are presented as mean and 95% CI. Differences between groups significant at P<0.05 are denoted by *.
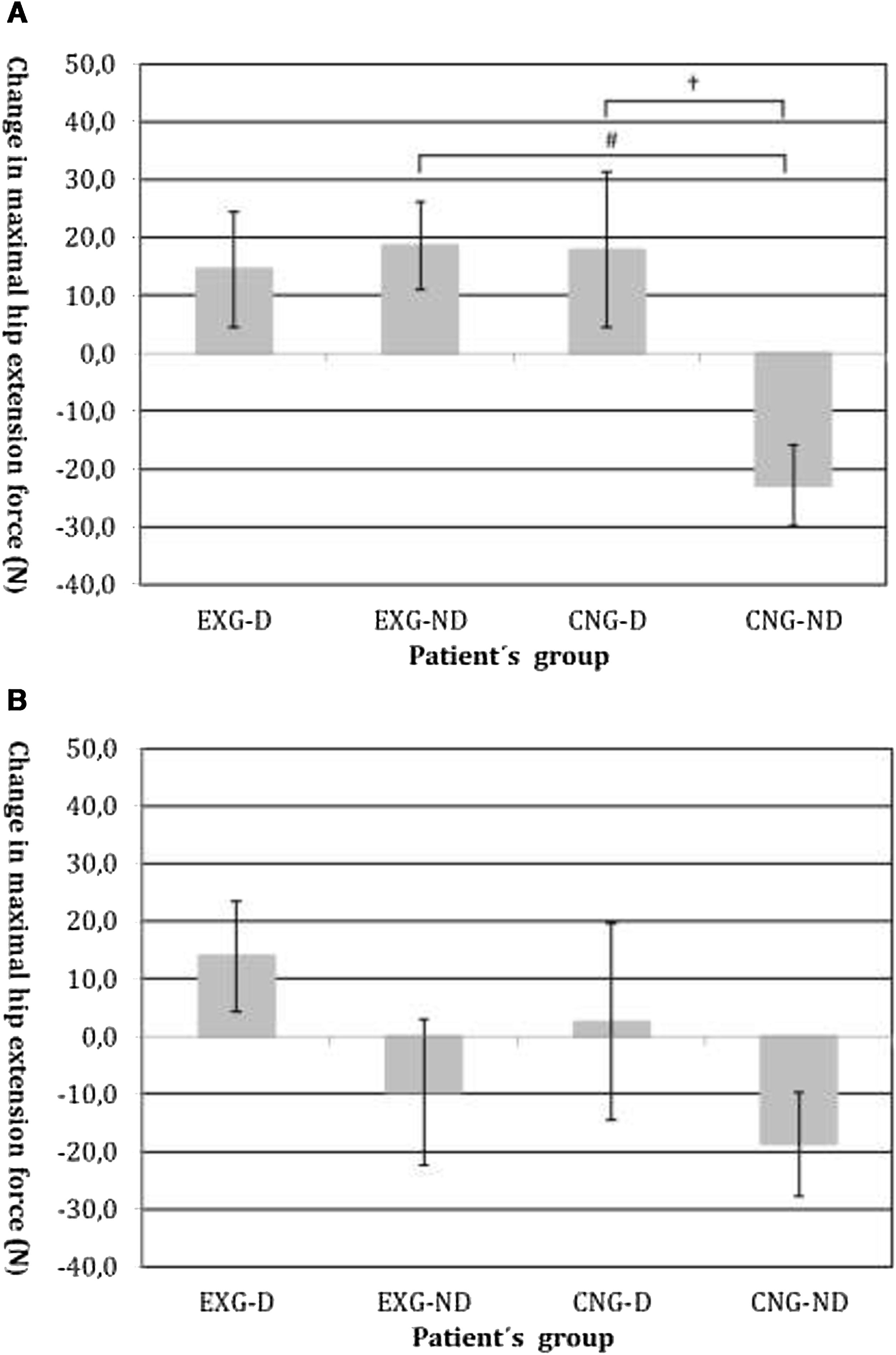
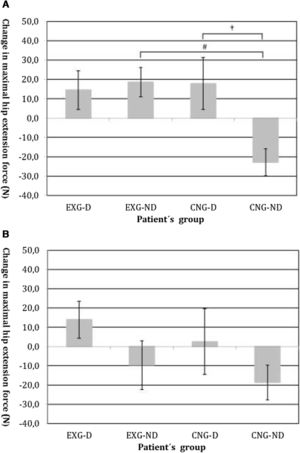
By an ANOVA pairwise comparison of data, we found that in the CNG group, the change of HE was significantly greater in the non-diabetic patients compared to the diabetic patients (difference 40.6, 95% CI=10.1 to 71.1, P<0.01). We also identified a significantly higher change in HE in EXG patients without diabetes compared to the CNG patients without diabetes (difference 41.3, 95% CI=20.6 to 62.1, P<0.001). We did not identify any significant difference in the change of HE between groups of patients after follow-up (Table 3). Changes in maximal forces during the HE intervention and after follow-up are graphically summarized in Fig. 2A and B, respectively.
(A) Changes in maximal isometric force during hip extension contractions after intervention, measured in Newtons (N). Groups are as follows: experimental diabetic patients group (EXG-D, N=11), experimental non-diabetic patients group (EXG-ND, N=19), control diabetic patients group (CNG-D, N=6) and control non-diabetic patients group (CNG-ND, N=22). Data represent the mean and error bars present the standard error of the mean. Differences between groups significant at P<0.01 are denoted by †. Differences between groups significant at P<0.001 are denoted by #. (B) Changes in maximal isometric force during hip extension contractions after follow-up, measured in Newtons (N). Groups are as follows: experimental diabetic patients group (EXG-D, N=11), experimental non-diabetic patients group (EXG-ND, N=19), control diabetic patients group (CNG-D, N=6) and control non-diabetic patients group (CNG-ND, N=22). Data represent the mean and error bars present the standard error of the mean.
We performed this study to assess the interactions between the presence of diabetes mellitus and a change in lower extremity muscle function among dialysis patients. From a total study sample of 90 patients enrolled at the start of the study, 64 patients completed the intervention and 58 completed the follow-up. The drop-outs from study sample occurred due to mortality, transplantations, serious infections, musculoskeletal issues and personal decision of patients. No adverse effects of the resistance training programme were noticed during the intervention. The mortality and drop-out rates were in a normal range and reflected the inclusion/exclusion criteria applied during the screening of the patients’ eligibility for participation on this study.
After the intervention, we found significant differences in the change of HE muscle function between non-diabetic EXG patients and non-diabetic CNG patients and also between diabetic and non-diabetic CNG patients. Generally, the application of IRT improved muscle function, and therefore the greater change in HE in EXG patients compared to CNG patients is not surprising.20,25
More interesting are the missing differences in the change of muscle function between diabetic and non-diabetic EXG patients and significant differences found in the change of muscle function between diabetic and non-diabetic CNG patients. Impaired glucose metabolism in diabetic patients has been associated with lower general fitness and with a reduced adaptability to exercise interventions.18,26 However, our results indicate that diabetic CKD-5D patients achieved similar improvements in muscle function during resistance training and that inactivity caused impairment in muscle function mainly in non-diabetic patients only and not in diabetic patients. There are several potential explanations for these interesting results. The majority of CKD-5D patients with diabetes mellitus are obese and this fact should be considered as metabolically- and nutritionally-protective factor for muscle mass and function among CKD-5D patients. Obese and overweight dialysis patients have higher body composition reserves and physical abilities compared to normal-weight dialysis patients according to BMI.27 Dialysis induced protein energy wasting and inflammation should negatively affect muscle function and adaptation to physical activity in normal-weight patients, and higher total muscle and fat volumes should mediate the higher potential for training adaptability among obese patients. This complex physiological phenomenon, called the “Obesity paradox”, was associated with better prognostics and surveillance in dialysis patients and may also contribute to the contradictory results in changes of muscle function found in our study.28,29 Another potential explanation of our results may be connected with differences in the adaptation of insulin metabolism after resistance training between diabetic and non-diabetic patients. There are consistent findings showing a decrease in insulin resistance in type 2 diabetic patients after physical exercise.30 Therefore, insulin resistance might play an important role in diabetics compared to non-diabetics and very likely mediate the effects of exercise and inactivity conditions on the musculoskeletal apparatus. Such a hypothesis should be tested in further research, e.g. comparing the Homeostatic Model Assessment for Insulin Resistance Index or hepatic elastography, or assessment of abdominal fat percentage between diabetics and non-diabetics patients. Unfortunately, our study was not designed to go deeper in this hypothesis. The increase of muscle functions found in inactive patients should be explained by behaviours and clinical variables. Both, increased attention from the research team members and improvements in clinical status related to maintenance dialysis therapy should contribute to positive changes found in muscle functions among inactive patients.
Our results show that the CKD-5D population is heterogeneous when evaluating the effectiveness of resistance training on muscle function. There are several factors that influence the effect of intradialytic resistance training on muscle function, and diabetic status is an important one. Some data also show substantial response variations in glucose homeostasis, insulin sensitivity and mitochondrial muscle density with approximately 15–20% of individuals failing to show improvement of a metabolic disorder with exercise.31
Our study has some important strengths. All assessments and intervention were realized during regular dialysis sessions and all methods were organized and modified to maximize patient safety while maintaining reasonable validity and the reliability of procedures. An important additional value of our research, contrary to other exercise intervention studies realized in dialysis care, is the methodology of muscle function assessments. During these assessments we used three different muscle function tests, leading us to more accurate and valid data about the change of muscle function during the experiment. The advantage of geographical allocation (with notable distance between allocations) is minimizing the effect of the intervention on the control subjects. Our study also has some limitations. Firstly, the allocation of CKD-5D patients into the study group was done in non-randomized fashion according to the geographical location of the dialysis centre location where patients receive dialysis therapy. This leads to differences in baseline clinical characteristics between the patient groups. We found significant differences in body mass index, dialysis adequacy and concentrations of albumin and calcium. To minimize the effects of differences in the management of participating dialysis centres, the selection of three dialysis locations was done based on similarities in patient capacities, staff size and quality management of the medical care provided. Secondly, our study is lacking the investigators’ blinding during intervention. The absence of blinding is typical for “exercise” intervention studies and cannot be completely avoided during the application of intradialytic training intervention.
ConclusionsAccording to existing evidence, considerable individual differences in physiological and functional adaptation to resistance training were found among dialysed patients compared to healthy and diabetic subjects. In our study, diabetic and non-diabetic patients responded positively to 12 weeks of intradialytic resistance training and improved their muscle function of lower extremities. We did not find significant differences in functional adaptation between diabetic and non-diabetic patients. Our findings indicate that exercise intervention during dialysis increases lower extremity muscle function regardless of the presence of diabetes. In the control condition, inactivity negatively affected muscle function only among non-diabetic control patients. Diabetic control patients improved their muscle function in similar extent compared to experimental patients. Physical inactivity negatively affected muscle function of haemodialysis patients especially among those with no presence of diabetes. Therefore, regular physical activity should be included in routine health care in dialysis centres.
FundingThis work was supported by the Slovak Research and Development Agency under Contract no. APVV-16-0490 and was supported by the Internal Research Grant System of Pavol Jozef Safarik University under Contract no. VVGS-2019-1069. The funders had no role in the study design, collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Conflicts of interestNone declared.
We appreciate the co-operation of the representatives and staff of dialysis centres involved in the implementation of this study. Special acknowledgements go to Peter Mizla MD and Peter Javorsky MD for their organizational support during the study planning.