Type 1 Diabetes Mellitus (DM1) is the second chronic disease and the most frequent endocrine-metabolic disorder in childhood. The objective of this study is to estimate direct cost of DM1 in pediatric patients in Andalusia.
MethodologyThis is a descriptive, multicenter observational study conducted during six months of 2017–2018, based on a sample of 220 patients, from 6 hospitals in Andalusia. Data from demographic and metabolic control variables, use of continuous glucose monitoring systems, average HbA1c, episodes of severe hypoglycaemia and ketoacidosis, comorbidities and complications, as well as direct health costs were collected; including costs of medication, materials, analytical determinations, complementary tests and costs derived from both in-hospital and out-of-hospital healthcare.
ResultsThe sample size was 178 patients. The average of diagnosis age was 6 years, and 4.69 (0.29 SD) years of disease evolution. Average HbA1c was 7.06%, 25% had a result above 7.5%. The estimated annual cost per patient was € 4,720.4. The cost derived from insulins (€ 2,212.9) and insulin administration materials and blood glucose monitor (€ 1,518) added the highest cost percentage (79.1%).
ConclusionThe study has shown a direct cost associated with DM in pediatric age in Andalusia of approximately € 4,700 per patient. Finally, an association between metabolic control, comorbidities or complications and the disease cost has not been detected.
La Diabetes Mellitus tipo 1 (DM1) es la segunda enfermedad crónica y el trastorno endocrino-metabólico más frecuente en la infancia. El objetivo de este estudio es realizar una estimación del coste directo de la DM1 en Andalucía, en pacientes pediátricos.
MetodologíaSe trata de un estudio descriptivo, observacional multicéntrico realizado durante seis meses consecutivos del 2017–2018, partiendo de una muestra de 220 pacientes, procedentes de 6 centros hospitalarios de Andalucía. Se recogieron variables demográficas, variables relacionadas con el control metabólico, uso de sistemas de monitorización continua de glucosa, hemoglobina glicosilada (HbA1c) media, episodios de hipoglucemias graves o cetoacidosis, comorbilidades y complicaciones existentes, así como los costes directos sanitarios; englobando los costes de medicación, materiales, determinaciones analíticas, pruebas complementarias y los relacionados con la asistencia sanitaria tanto hospitalaria como extrahospitalaria.
ResultadosSe obtuvo una muestra de 178 pacientes. La edad media al diagnóstico fue de 6 años y los años de evolución de la enfermedad de 4.69 (0.29 DE) años. La HbA1c media fue de 7.06%, encontrándose el 25% por encima de 7.5%.El coste medio anual estimado por paciente fue de 4,720.4 €. El derivado de las insulinas (2,212.9€) y el material para la administración de la misma y monitorización de la glucemia (1,518 €), supusieron el mayor porcentaje del gasto (79.1%).
ConclusiónEste estudio demuestra un coste directo asociado a la DM en edad pediátrica en Andalucía de aproximadamente 4,700€ por paciente. No se detecta asociación entre el control metabólico, comorbilidades y el coste de la enfermedad.
Type 1 diabetes mellitus (DM1) is the second most common chronic disease and the most frequent endocrine-metabolic disorder in childhood. In Spain, the estimated prevalence of DM1 is 1.1–1.4 cases per 1000 people in the population under 15 years of age. These patients in turn represent 5–10% of the global population with diabetes. However, in the region of Andalusia the minimum prevalence is 1.7 cases per 1000 people, i.e., comparatively higher than the national average.1 Type 1 diabetes poses an important economic burden due to its prevalence, the considerable use of resources associated with the management of the disease (treatment device alternatives, follow-up visits to different healthcare professionals, etc.), and the short and long term complications that may occur in such patients.
The study conducted in Andalusia in the period 2000–2009 reported an incidence of DM1 in children under 14 years of age of 20.76 cases per 100,000 inhabitants/year. For the Andalusian province of Málaga, López-Siguero2 reported an annual incidence of 16.5 cases per 100,000 inhabitants/year. Likewise, Conde-Barreiro3 conducted a comparative analysis of the incidence and prevalence of the disease in the different Spanish Autonomous Communities. Broad geographical variability was observed, without the "north-south gradient" of incidence described in the studies conducted elsewhere in Europe. The data from Andalusia were subsequently updated in 2014, with an incidence of 325 children under 14 years of age and 2550 patients with DM1 under 14 years of age (a prevalence of 17 cases per 100,000 inhabitants).
In recent years, major advances and numerous incorporations of new healthcare technologies have facilitated good metabolic control, such as continuous subcutaneous insulin infusion (CSII) systems or different interstitial glucose monitoring devices. In Europe, the Working to Create Centers of Reference registry in 2015 reported the proportion of patients receiving CSII to be 40.2%.4,5 The data available in Spain report the percentage use of CSII in diabetic patients under 15 years of age to be approximately 12%. Likewise, 50% of the centres claim to have used some continuous glucose monitoring system.6 However, these figures differ greatly from the situation in Andalusia, where only 5.5% of the patients use CSII systems according to the 2014 study on the situation of diabetes mellitus in this Spanish Autonomous Community.1
The increase in prevalence of the disease and the introduction of new insulins and technologies on the market have increased the costs associated with diabetes. However, very few studies can be found in the literature on the cost of DM1 in pediatric patients. In addition, there are many differences in the methodology used, the inclusion criteria applied, patient age, the time horizon, and the care setting (private or public).7–10 In this context, mention should be made of the CHRYSTAL trial, a multicenter observational study conducted at 12 centres in Spain, in which a sample of 249 pediatric patients was analysed. The results of this study estimated a total annual cost of 27,274 €/patient/year (data obtained in 2014), including the direct healthcare and non-healthcare costs.11
The objectives of the present study were to estimate the direct cost per patient associated with DM1 in the pediatric population in Andalusia, analysing its variation according to the degree of metabolic control and the type of insulin therapy used, and to compare our results with those published to date at both the national and international level.
MethodologyStudy designA prospective, descriptive, observational multicenter study with a time horizon of 6 months (February to July 2017) was carried out, with subsequent extrapolation to one year. A sample size of 220 cases was calculated in order to secure a precision of 5.3% in the estimation of a proportion, with a normal asymptotic two-tailed 95% confidence interval, and assuming the proportion of the annual cost in hospitalizations related to diabetes to be 32%. The inclusion criteria were patients aged 2–16 years, with a time since diagnosis of the disease of at least one year, and whose parents or legal guardians had signed an informed consent to participation in the study. Patients diagnosed with other types of diabetes or with some other physical disease or limitation that could bias the results because of increased costs due to the association of different diseases were excluded. The study was approved by the Ethics Committee of Hospital Regional Universitario de Málaga, from where the entire study was coordinated. After excluding those patients whose parents or legal guardians had failed to adequately fill in the questionnaire, we obtained a final sample of 178 patients.
The cases were distributed in a randomised manner and proportional to the number of pediatric patients seen in the pediatric endocrinology outpatient clinics of each of the 6 hospitals in Andalusia selected to participate in the study: Hospital Serranía de Ronda, Hospital General San Agustín de Linares, Hospital Materno-infantil de Jaén, Hospital Materno-infantil de Jerez, Hospital Virgen Macarena de Sevilla and Hospital Materno-infantil de Málaga. The patient cohort was segmented based on age (2–4 years, 5–7 years, 8–12 years and 13–16 years) to ensure a homogeneous sample according to the prevalence of the disease.
Characteristics of the study populationStarting from 220 patients, and after excluding those who did not meet the pre-established requirements (non-compliance with the inclusion criteria or failure to adequately fill in the questionnaire), a final sample of 178 patients was obtained.
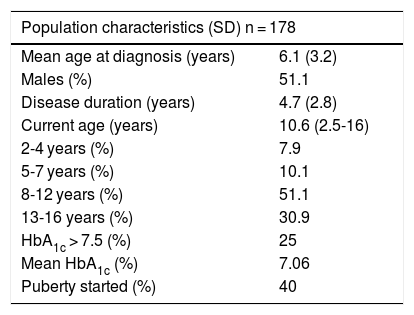
A total of 51.1% of the included subjects were males, and the mean age at diagnosis was 6.1 years. Sixty-seven percent had suffered the disease for more than 5 years, and the mean duration was 4.7 years. The mean patient age at the time of the survey was 10.6 years (Table 1).
Characteristics of the study population.
| Population characteristics (SD) n = 178 | |
|---|---|
| Mean age at diagnosis (years) | 6.1 (3.2) |
| Males (%) | 51.1 |
| Disease duration (years) | 4.7 (2.8) |
| Current age (years) | 10.6 (2.5-16) |
| 2-4 years (%) | 7.9 |
| 5-7 years (%) | 10.1 |
| 8-12 years (%) | 51.1 |
| 13-16 years (%) | 30.9 |
| HbA1c > 7.5 (%) | 25 |
| Mean HbA1c (%) | 7.06 |
| Puberty started (%) | 40 |
SD: standard deviation.
Continuous subcutaneous insulin infusion (CSII) systems were used by 12.9% of the patients, and an interstitial glucose monitoring device was used by 16.8%. Of the latter patients, 9.5% employed the standard on-demand flash measuring device, while 7.3% used a real-time continuous glucose monitoring (RT-CGM) system integrated with CSII.
QuestionnairesThe questionnaires were completed by the parents or legal guardians while they were in the waiting room. The following variables were recorded:
- -
Demographic parameters: patient age at diagnosis, disease duration, gender and pubertal stage.
- -
Information related to metabolic control of the patients (data collected from the review of previous episodes in the electronic database): treatment alternatives (multiple insulin doses [MDI] or CSII), as well as the use of on-demand flash glucose measuring devices and RT-CGM. The degree of metabolic control of the patients with DM1 was estimated based on the mean value of the two previous glycosylated hemoglobin (HbA1c) measurements (capillary and/or venous blood). Lastly, we documented the episodes of severe hypoglycemia occurring during the previous year (defined as episodes which the patient was unable to resolve on his/her own and that required the intervention of a third person), episodes of diabetic ketoacidosis (DKA), and possible comorbidities associated with diabetes.
From the collected data, we analysed the associated direct healthcare costs. The costs generated by healthcare in the different settings were recorded prospectively. For this purpose, the estimated costs of each medical intervention were used, employing the public rates corresponding to the healthcare services provided by the medical centers dependent upon the Andalusian public health system.12
Both inpatient (hospital) and outpatient direct healthcare costs were included. The hospital costs included visits by specialists (endocrinology, ophthalmology and mental health) and nursing staff, emergency room visits for diabetes-related reasons, and hospital admissions. All information related to emergency room visits and hospital admissions was obtained from the electronic system used in Andalusia. The outpatient costs in turn included visits by primary care pediatricians for diabetes-related reasons and nursing staff at the medical centre, as well as visits to community mental health and peripheral specialty centres. Data on the amount of insulin used were collected from the pharmaceutical dispensation of vials and pens. The use of concomitant medication was also obtained from the pharmaceutical prescriptions.
The costs associated with the explorations and laboratory tests performed (blood and urine tests, and HbA1c measurements) were obtained from the data of Hospital Regional de Málaga, and served as the mean reference standard. Similarly, those related to healthcare were assigned following the provisions of the hospital administration, based on the data compiled in BOJA (Official Bulletin of the Government of Andalusia) number 218 of 2016. The method used for measuring HbA1c was DCA 2000 followed by the Advantage technique - the inhibition of latex particle immunoagglutination assay.
All costs were updated to 2018 euros according to the corresponding Consumer Price Index (CPI) variation.
Sensitivity analysisThe R commander application was used for the statistical analysis of the data. A base case was considered to estimate the total cost associated with the management of the disease in the selected patient cohort. In addition, a number of sensitivity analyses were made to examine the impact of the variations of the following variables upon the total cost: HbA1c levels (for an HbA1c of over 7.5%) and alternative treatment with MDI or CSII. In order to analyse the potential association between elevated HbA1c and increased cost, hypothesis testing (Student t-test, analysis of variance [ANOVA], chi-squared test) was used according to the type of variable involved.
ResultsThe results of the present study showed the mean HbA1c concentration in the patient cohort to be 7.1%, with values of over 7.5% in 25% of the cases. The mean number of annual HbA1c measurements was 3.6 per patient (in both capillary and venous blood). The mean number of routine laboratory tests performed per year was 1.6, with assessment of thyroid function and screening for celiac disease.
A total of 5.5% of the patients suffered a severe hypoglycemia episode, and 1.1% experienced a diabetic ketoacidosis (DKA) episode during the previous year. In view of the short time from the onset of the disease, only 1.1% of the patients presented renal complications in the form of microalbuminuria, while 8.4% and 8.3% had celiac disease and autoimmune thyroiditis, respectively.
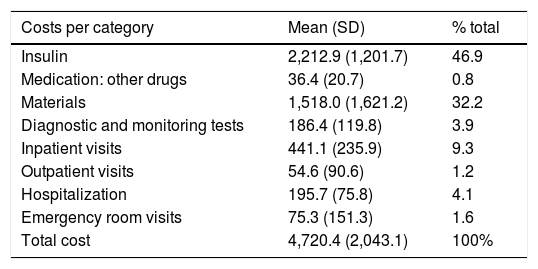
The estimated total cost was 4720.4 € on average. Insulins (slow- and fast-acting) were the item with the strongest impact upon the total cost (2212.9 €, representing 46.9% of the total). The total pooled cost, taking into account insulin, other drugs (mainly glucagon and levothyroxine) and materials (blood glucose and ketonemia strips, insulin infusion and glucose monitoring systems) was 3767.3 € annually per patient. The cost associated with diagnostic tests (the measurement of HbA1c, microalbuminuria, radiographs, general laboratory tests including complete blood count, biochemistry with ion profile, hormone tests, thyroiditis and celiac disease antibodies, and calprotectin in stools) was 186.4 €. Lastly, the cost associated with inpatient (hospital)(endocrinology, nursing, social workers, ophthalmology) and outpatient care (in primary care, both pediatrics and nursing), including hospital admissions and emergency room visits, totalled 766.7 € (Table 2).
Mean cost per patient per year in 2018.
| Costs per category | Mean (SD) | % total |
|---|---|---|
| Insulin | 2,212.9 (1,201.7) | 46.9 |
| Medication: other drugs | 36.4 (20.7) | 0.8 |
| Materials | 1,518.0 (1,621.2) | 32.2 |
| Diagnostic and monitoring tests | 186.4 (119.8) | 3.9 |
| Inpatient visits | 441.1 (235.9) | 9.3 |
| Outpatient visits | 54.6 (90.6) | 1.2 |
| Hospitalization | 195.7 (75.8) | 4.1 |
| Emergency room visits | 75.3 (151.3) | 1.6 |
| Total cost | 4,720.4 (2,043.1) | 100% |
SD: standard deviation.
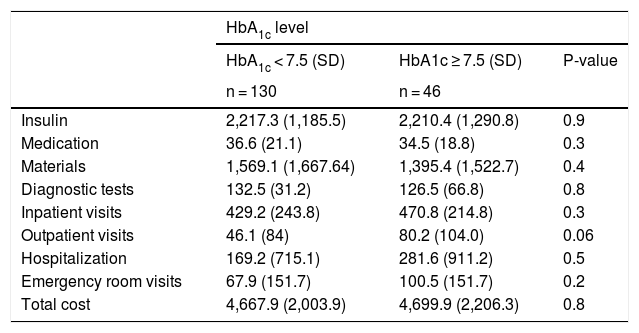
Table 3 details the mean annual cost per patient according to the HbA1c level. Also shown are the costs by categories: the cost of insulin (basal and fast-acting analogs), other medications, material costs, diagnostic tests, hospital visit costs, outpatient visit costs, hospitalization and emergency room care. With regard to the number of hospital admissions, there were no significant differences between the group of patients with HbA1c > 7.5% (6.5%) and those with better glycemic control (5.3%). The results of our analysis suggest that there were no differences in the above-mentioned patient subgroups.
Mean cost per patient per year in 2018 classified according to HbA1c level.
| HbA1c level | |||
|---|---|---|---|
| HbA1c < 7.5 (SD) | HbA1c ≥ 7.5 (SD) | P-value | |
| n = 130 | n = 46 | ||
| Insulin | 2,217.3 (1,185.5) | 2,210.4 (1,290.8) | 0.9 |
| Medication | 36.6 (21.1) | 34.5 (18.8) | 0.3 |
| Materials | 1,569.1 (1,667.64) | 1,395.4 (1,522.7) | 0.4 |
| Diagnostic tests | 132.5 (31.2) | 126.5 (66.8) | 0.8 |
| Inpatient visits | 429.2 (243.8) | 470.8 (214.8) | 0.3 |
| Outpatient visits | 46.1 (84) | 80.2 (104.0) | 0.06 |
| Hospitalization | 169.2 (715.1) | 281.6 (911.2) | 0.5 |
| Emergency room visits | 67.9 (151.7) | 100.5 (151.7) | 0.2 |
| Total cost | 4,667.9 (2,003.9) | 4,699.9 (2,206.3) | 0.8 |
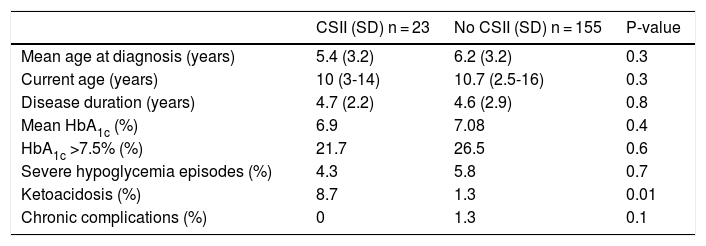
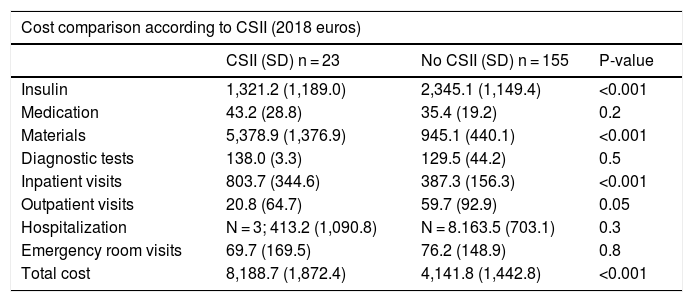
Tables 4 and 5 detail the baseline characteristics and other demographic variables and associated costs according to whether or not the patients were receiving CSII. No differences were found in HbA1c levels or other demographic variables, though an increased incidence of DKA was noted in the CSII group. The total cost was lower in the MDI versus CSII patient group, being 4141.8 € and 8188.7 €, respectively. The results suggest a decreased insulin cost in patients with CSII as a consequence of lesser insulin requirements in these individuals (2345.1 € versus 1321.2 € for MDI and CSII, respectively). The group of patients with CSII generated greater material costs (5378.9 €), this comprising all consumables referring to the infusion system and the material for real-time continuous glucose monitoring (RT-CGM). The proportion of admissions in patients with CSII was 13%, as compared to 5% in those with MDI, and this resulted in a higher cost per admission among the patients with CSII. However, this finding is biased by the lower proportion of patients with CSII (i.e., expense divided by fewer patients); the two groups are therefore not comparable. It is therefore important to clarify that both the costs and causes, and the mean length of stay, were similar in both groups.
Comparative use of CSII.
| CSII (SD) n = 23 | No CSII (SD) n = 155 | P-value | |
|---|---|---|---|
| Mean age at diagnosis (years) | 5.4 (3.2) | 6.2 (3.2) | 0.3 |
| Current age (years) | 10 (3-14) | 10.7 (2.5-16) | 0.3 |
| Disease duration (years) | 4.7 (2.2) | 4.6 (2.9) | 0.8 |
| Mean HbA1c (%) | 6.9 | 7.08 | 0.4 |
| HbA1c >7.5% (%) | 21.7 | 26.5 | 0.6 |
| Severe hypoglycemia episodes (%) | 4.3 | 5.8 | 0.7 |
| Ketoacidosis (%) | 8.7 | 1.3 | 0.01 |
| Chronic complications (%) | 0 | 1.3 | 0.1 |
SD: standard deviation; CSII: continuous subcutaneous insulin infusion.
Comparison of mean cost per patient per year in 2018 according to CSII.
| Cost comparison according to CSII (2018 euros) | |||
|---|---|---|---|
| CSII (SD) n = 23 | No CSII (SD) n = 155 | P-value | |
| Insulin | 1,321.2 (1,189.0) | 2,345.1 (1,149.4) | <0.001 |
| Medication | 43.2 (28.8) | 35.4 (19.2) | 0.2 |
| Materials | 5,378.9 (1,376.9) | 945.1 (440.1) | <0.001 |
| Diagnostic tests | 138.0 (3.3) | 129.5 (44.2) | 0.5 |
| Inpatient visits | 803.7 (344.6) | 387.3 (156.3) | <0.001 |
| Outpatient visits | 20.8 (64.7) | 59.7 (92.9) | 0.05 |
| Hospitalization | N = 3; 413.2 (1,090.8) | N = 8.163.5 (703.1) | 0.3 |
| Emergency room visits | 69.7 (169.5) | 76.2 (148.9) | 0.8 |
| Total cost | 8,188.7 (1,872.4) | 4,141.8 (1,442.8) | <0.001 |
SD: standard deviation; CSII: continuous subcutaneous insulin infusion.
With regard to the statistical results, no significant association was found between HbA1c > 7.5% and a greater number of admissions, or even increased direct healthcare costs associated with the management of the disease, as commented above.
DiscussionThe results obtained in our study show the patient cohort to have had good metabolic control, with HbA1c < 7.5% in 75% of the cases, as well as a low incidence of acute and chronic complications.
As regards the direct healthcare costs associated with the management of the disease, the results were very homogeneous in the different subgroups studied, regardless of HbA1c concentration.
The direct healthcare costs (4720.4 €) were slightly higher than in the CHRYSTAL study11 (4070.08 €; euros corresponding to the year 2014), in which there was a greater difference between the two groups (HbA1c < 7.5% and ≥ 7.5%). This difference may be a consequence of the fact that the mean HbA1c level of the population enrolled in our study was slightly lower than in the CHRYSTAL trial (7.06% versus 7.42%). Among the direct healthcare costs we observed a higher expense in insulin and medication, and a lower cost in materials. The costs of inpatient and outpatient visits, as well as the costs of hospitalization, were lower than in the CHRYSTAL study, possibly due to differences in the method used to compile the number of visits. In the latter study, the authors asked about the care received in the final three months and extrapolated the situation to one year, while in our case we reviewed each of the visits of each patient over the previous year. Lastly, the costs derived from emergency care were very similar in both studies.
The main difference between the methodology used in our study and the approach adopted by the CHRYSTAL trial was that the latter identified, estimated and quantified resource consumption associated with direct non-health care costs (including family expenses on transport, special food and physical activities, among other things) and the costs associated with informal care provided by non-professional caregivers who performed tasks concerned with maintaining or improving patient health. The costs derived from this latter group represented a much larger proportion of the total cost.
To the best of our knowledge, this is the first study to estimate the direct healthcare cost of DM1 in pediatric patients in Andalusia. Different studies have been published on the economic burden of DM1 in pediatric patients, with differences conditioned by the healthcare systems present in the different countries, as well as by the great diversity of the methodological approaches used. A study in Greece13,14 estimated an average annual direct cost of 2712 € per patient. In the United Kingdom15 the data suggested an average annual cost of 4744 € per patient. The ENTRED 2007 study, conducted in France,16 reported 6927 € per patient per year, though on excluding hospitalization costs associated with the start of the disorder, the figure decreased to 4329 € per patient per year. In Germany,17 the estimated average annual cost was 3524 € per patient, while in the United States18,19 the reported figure was 4730 € per patient per year. Therefore, all these studies report annual direct costs that are quite similar to those obtained in our study. As commented above, the differences are probably due to the different data compilation methods used and the variability in costs conditioned by each particular healthcare system.
The present study is not without limitations. The selected patients had a short duration of the disease (<15 years), and were therefore not yet expected to present chronic complications secondary to diabetes. Likewise, the small sample size of the subgroups, particularly the subgroup of individuals with SCII (23 patients), implied a lack of statistical power for detecting significant differences. In addition, the utilization of HbA1c as a measure to assess metabolic control may be insufficient, and other parameters such as glycemic variability (not measured in our study) should be added. Lastly, resource utilization associated with informal care was not documented, and it was therefore impossible to quantify the direct non-healthcare costs related to the management of the disease.
Regarding the implementation of CSII, mention should be made of the article published in 2017 on the situation of diabetes mellitus in Andalusia, where a prevalence of CSII of 5.5% was reported.1 However, in our study we recorded a prevalence of 12.9%, which is more consistent with the existing literature. In this regard the SWEET Registry,4,5 which evaluated the use of CSII in 48 centres, recorded a prevalence of 40.2%, while in Greece13,14 the reported figure was 10%. All of this suggests the possibility of underestimation of the use of CSII in Andalusia. We therefore recommend that a national and international registry be set up to estimate and update on the introduction of this therapy in the different regions and/or countries. However, it appears that the use of new technologies is increasing, and the associated costs are therefore also increasing. It may therefore be worthwhile to assess the possibility of incrementing the financial resources assigned to the management of this disease.
Similarly, on comparing the patients treated with CSII versus those receiving MDI, a higher cost was recorded in the former group, as well as a higher incidence of DKA. This could cause us to reconsider the efficacy of this therapeutic modality. However, many studies have shown improved metabolic control in pediatric patients treated with CSII,5 with a decrease in HbA1c concentration, as well as lower total daily insulin requirements.
All this reflects the need for more studies and registries on this frequent disease in childhood, as well as the considerable advance in the incorporation of new devices and therapies in recent years that may substantially modify the direct costs associated with the care of these patients.
ConclusionsThe direct cost associated with DM1 in children in Andalusia is approximately 4700 € per patient per year. On analysing the use of CSII or MDI, no association was found between the degree of metabolic control and the cost of the disease.
Comparison of the costs associated with the disease with other countries yielded very similar results. All studies agree that there is an increase in cost due to the implementation of new technologies, including CSII. Despite the abovementioned limitations, our study demonstrates the importance of analysing different population cohorts with DM1, not only for determining the degree of metabolic control of the disease and/or the potential complications, but also for estimating the costs associated with the new technologies, with a view to facilitating informed decision-making.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Álvarez Casaño M, Alonso Montejo MM, Leiva Gea I, Jiménez Hinojosa JM, Santos Mata MÁ, Macías F, et al. Estudio de costes directos de la diabetes mellitus tipo 1 en pacientes entre 2 y 16 años en Andalucía. Endocrinol Diabetes Nutr. 2019;66:480–486.