Low prolactin levels have been found to impair libido and arousal, as well as to reduce wellbeing in young women.
ObjectiveThe aim of this study was to investigate whether drug-induced hypoprolactinaemia affects male sexual function and depressive symptoms.
MethodsThe study population consisted of three groups of young and middle-aged men. Two groups were treated with dopamine agonists because of previous hyperprolactinaemia but differed in current prolactin levels, which were <3ng/ml (n=12; group 1) or within the reference range (3–20ng/ml) (n=20; group 2). The control group (group 3) included 24 dopamine agonist-naïve normoprolactinaemic men. During the study, doses of dopaminergic agents in group 1 were reduced by 25–50% compared to doses before the start of the study. Circulating levels of prolactin, testosterone, free calculated testosterone, dehydroepiandrosterone-sulphate, oestradiol and gonadotropins were measured upon enrolment in the study and six months later. Moreover, at the beginning and the end of the study, all men enrolled completed questionnaires assessing sexual functioning (IIEF-15) and depressive symptoms (BDI-II).
ResultsGroup 1 differed from groups 2 and 3 in domain scores for sexual desire and erectile function, and in the overall BDI-II score. It was also characterised by lower levels of total testosterone and calculated free testosterone. Reduction of drug doses normalised sexual desire and erectile function, reduced BDI-II scores and increased prolactin as well as total and free calculated testosterone. Groups 2 and 3 did not differ from each other in sexual functioning, depressive symptoms or hormone levels.
ConclusionsThe results obtained indicate that men with dopamine agonist-induced hypoprolactinaemia are characterised by impaired sexual functioning and reduced wellbeing. These disturbances are a consequence of subnormal prolactin levels and do not seem to reflect adverse effects of dopamine agonists.
Se ha descrito que los niveles bajos de prolactina perjudican la libido y la excitación, además de deteriorar el bienestar de las mujeres jóvenes.
ObjetivoEl objetivo de este estudio fue investigar si la hipoprolactinemia inducida por fármacos afecta la función sexual masculina y los síntomas depresivos.
MétodosLa población de estudio consistió en tres grupos de hombres jóvenes y de mediana edad. Dos grupos fueron tratados con agonistas dopaminérgicos debido a hiperprolactinemia previa, pero diferían en los niveles presentes de prolactina, que eran inferiores a 3 ng/mL (n = 12; grupo 1) o dentro del rango de referencia (3-20 ng/mL) (n = 20; grupo 2). El grupo de control (grupo 3) incluyó a 24 hombres normoprolactinémicos que no habían recibido agonistas dopaminérgicos. Durante el estudio, las dosis de agentes dopaminérgicos en el grupo 1 se redujeron en un 25-50%, en comparación con las utilizadas antes del inicio del estudio. Los niveles circulantes de prolactina, testosterona, testosterona libre calculada, sulfato de dehidroepiandrosterona, estradiol y gonadotropinas se midieron al inicio del estudio y seis meses después. Además, al comienzo y al final del estudio todos los hombres incluidos completaron cuestionarios que evaluaban el funcionamiento sexual con el índice internacional de función eréctil (IIEF-15), y los síntomas depresivos con el inventario de depresión de Beck (BDI-II).
ResultadosEl grupo 1 difirió de los grupos 2 y 3 en las puntuaciones de los dominios para el deseo sexual y la función eréctil, y en la puntuación general de BDI-II, y se caracterizó por valores más bajos de testosterona total y testosterona libre calculada. La reducción de la dosis del fármaco normalizó el deseo sexual y la función eréctil, redujo la puntuación BDI-II y aumentó la prolactina, la testosterona calculada total y libre. Los grupos 2 y 3 no se diferenciaron entre sí en cuanto a funcionamiento sexual, síntomas depresivos y niveles hormonales.
ConclusionesLos resultados obtenidos indican que los hombres con hipoprolactinemia inducida por agonistas dopaminérgicos se caracterizan por un funcionamiento sexual deteriorado y un bienestar reducido. Estas alteraciones son una consecuencia de los niveles de prolactina por debajo de lo normal y no parecen reflejar los efectos adversos de los agonistas dopaminérgicos.
Chronic prolactin excess seems to exert a negative effect on male sexual functioning.1–4 The risk of significant hypoactive sexual desire was increased in men in whom circulating prolactin levels exceeded 35ng/ml.1 This risk, however, was not as high as in men with low testosterone levels.2 Hyperprolactinaemia was frequently reported in men with erectile dysfunction,3 while methadone-induced hyperprolactinaemia impaired male erectile and orgasmic function.4 Moreover, drug treatment of hyperprolactinaemia resulted in increased numbers of erections,5,6 while the prolactin-lowering effect of cabergoline was accompanied by improved libido.7 Interestingly, unlike in chronic hyperprolactinaemia, acute changes in prolactin secretion seem to play a role in regulation of sexual response, as a component of a negative feedback loop that ultimately reduces sexual arousal.8–10 Prolactin surges are regarded as a neurohormonal index of sexual satiety and an objective index of orgasm and orgasm quality.8–10
In recent years, studies have clearly shown that men treated with high doses of dopamine agonists may develop impulse control disorders such as gambling, compulsive shopping, binge eating and hypersexuality. Initially, cases of impulse control disorders were reported in subjects receiving these agents because of Parkinson's disease or restless leg syndrome.11,12 Hypersexuality may, however, also develop in men with prolactin-secreting tumours managed with dopamine agonists and usually resolve after treatment cessation; this is known as “dopa-testotoxicosis”.13,14 Because all participants in those studies had circulating prolactin levels within the reference range, increased libido was attributed to a stimulatory effect of dopamine agonists on dopaminergic transmission in the mesolimbic system, which activates the reward system.13,14 However, it was recently found that young women with prolactin levels below 5ng/ml were characterised by abnormally low sexual desire and arousal.15 Unfortunately, no corresponding data are available for men. Therefore, the aim of this study was to investigate whether low prolactin levels resulting from dopamine agonist treatment determine male sexual functioning and depressive symptoms.
MethodsThis study was approved by the Independent Ethics Committee and conducted in accordance with the Declaration of Helsinki in its latest revision. All enrolled patients provided written informed consent after receiving an explanation of the voluntary nature of their participation and a description of the study.
PatientsThe participants in this prospective cohort study were enrolled between January 2018 and August 2019. All participants were men (30–65 years old) supervised and/or treated with dopaminergic agents by local healthcare providers cooperating with our research team. The aim of treatment in all participants was to normalise prolactin levels, and the participants were unaware of their prolactin status. Drug choice and dosage were at the sole discretion of cooperating physicians. All individuals were invited by the investigators to take part in the study assessing their sexual functioning. The study population consisted of three groups. Groups 1 and 2 had been treated for at least four months with dopamine agonists because of previously diagnosed elevated prolactin levels (20–100ng/ml determined on at least two different occasions), secondary to traumatic brain injury, secondary to empty sella syndrome or idiopathic. Subjects were allocated into one of these groups based on prolactin levels on the first day of the study. Group 1 (n=12) included men with plasma prolactin levels below 3ng/ml, while group 2 (n=20) enrolled patients with plasma levels of this hormone within the reference range (3–20ng/ml). Hypoprolactinaemia in group 1 was an unintentional consequence of using overly high doses of cabergoline or bromocriptine. Six patients (50%) in group 1 and 11 patients (55%) in group 2 were treated with bromocriptine (2.5–15.0mg daily); the remaining patients were treated with cabergoline (0.25–1.5mg weekly). The degree of hyperprolactinaemia determined the doses of both drugs but not the group allocation. Group 3 included 24 men with prolactin levels within the reference range who had not been treated with dopaminergic agents. This group was selected from a larger group of normoprolactinaemic men (n=59) diagnosed because of a personal history of empty sella syndrome, head trauma or infertility. Screening was done on the basis of a computer algorithm intended to yield study arms matched for age, body mass index, smoking and blood pressure. To minimise the impact of seasonal fluctuations on sexual functioning, depressive symptoms and hormone levels, 28 patients were recruited in January and February and the remaining 26 patients were recruited in July or August.
The exclusion criteria were as follows: adrenal disorders, osteoporosis, hypogonadism, psychiatric disorders, kidney or liver failure, chronic infection, diabetes, abnormal rectal bleeding, developmental or acquired male reproductive system abnormalities, history of myocardial infarction or acute cerebrovascular events, history of total or transurethral prostatectomy, history of major pelvic surgery or other operations that might have affected sexual function, and sexual inactivity. Subjects treated with drugs known to either modulate sexual functioning and mood or affect prolactin levels were also excluded.
Study designIn order to normalise prolactin levels in group 1, at the beginning of the study, the dose of cabergoline and bromocriptine was reduced by 25%. If, four weeks later, prolactin levels were still below 3ng/ml, the dose was decreased to half of the initial dose. In group 2, dopamine agonists were administered at the same doses as before the beginning of the study, while group 3 did not receive any dopaminergic agent. During the entire study period (six months), the addition of new drugs was not allowed. Drug adherence was measured by pill count.
Laboratory testsAll experiments were conducted in duplicate by a person blinded to the study protocol. Venous blood samples were collected between 7:00AM and 8:30AM upon enrolment in the study and six months later. Blood samples were taken shortly (60–120min) after waking up, in a fasting state, after the patient had been resting in a seated position for at least 30min. Participants were asked to avoid stress, increased physical activity and sexual intercourse at least 12h before blood collection. Because of the pulsatile nature of prolactin secretion, prolactin levels were assessed in three blood samples obtained at 15-min intervals and end result was averaged. Plasma levels of prolactin, total testosterone, dehydroepiandrosterone-sulphate (DHEA-S), oestradiol, follicle-stimulating hormone (FSH), luteinising hormone (LH) and sex hormone-binding globulin (SHBG) were assayed by direct chemiluminescence using acridinium ester technology (ADVIA Centaur XP Immunoassay System, Siemens Healthcare Diagnostics, Munich, Germany). Plasma albumin and creatinine levels were assessed using reagents from Roche Diagnostics (Basel, Switzerland). Free testosterone was calculated from plasma levels of testosterone, SHBG and albumin using the Vermeulen equation16,17 and a free online testosterone calculator (www.issam.ch/freetesto.htm). Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) Study equation.
QuestionnairesJust after blood samples were taken, all subjects were asked to complete three questionnaires assessing: the sociodemographic characteristics of the study population, sexual functioning and depressive symptoms. The details of the questionnaires and their analysis have been previously reported.18–21
Statistical analysisAll endpoints were log-transformed to normalise their distributions. The study arms and differences between percent changes from baseline after adjustment for baseline values (reflecting dopamine agonist strength) were compared using one-way analysis of covariance followed by Bonferroni post hoc tests after consideration of age, body mass index, smoking and blood pressure as potential confounding factors. Differences between values within a single study arm were compared using one-way within-subjects analysis of covariance followed by Student's paired t test. The χ2 test was employed to compare proportional data. Pearson's r tests were used to test associations between endpoints. Differences were regarded as statistically significant if p values corrected for multiple testing using Benjamini and Hochberg's false discovery rate were below 0.05.
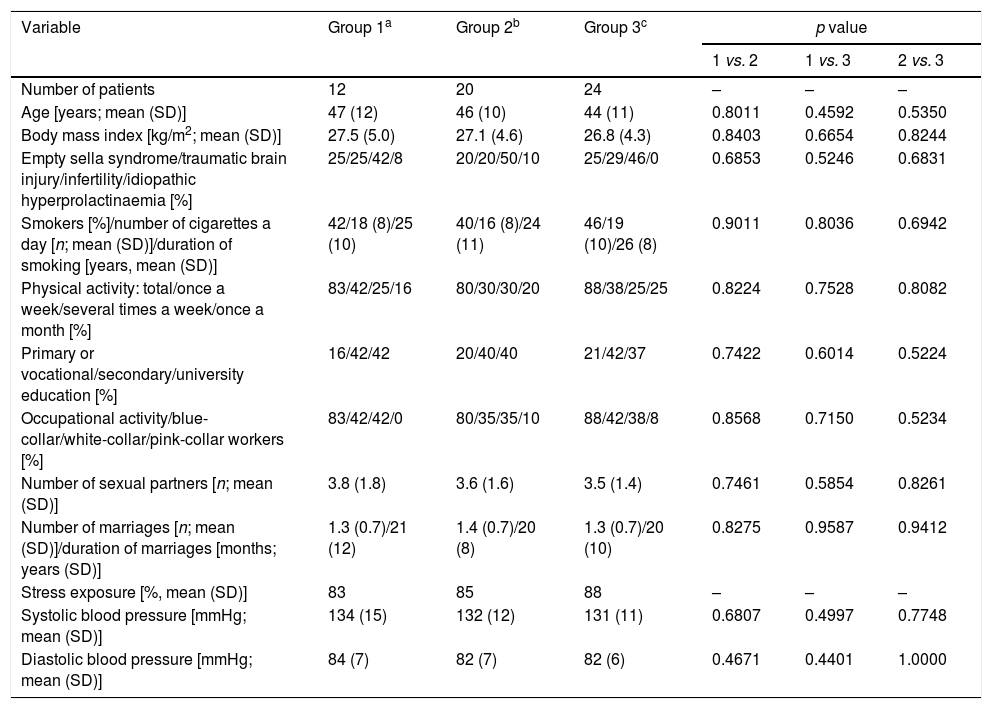
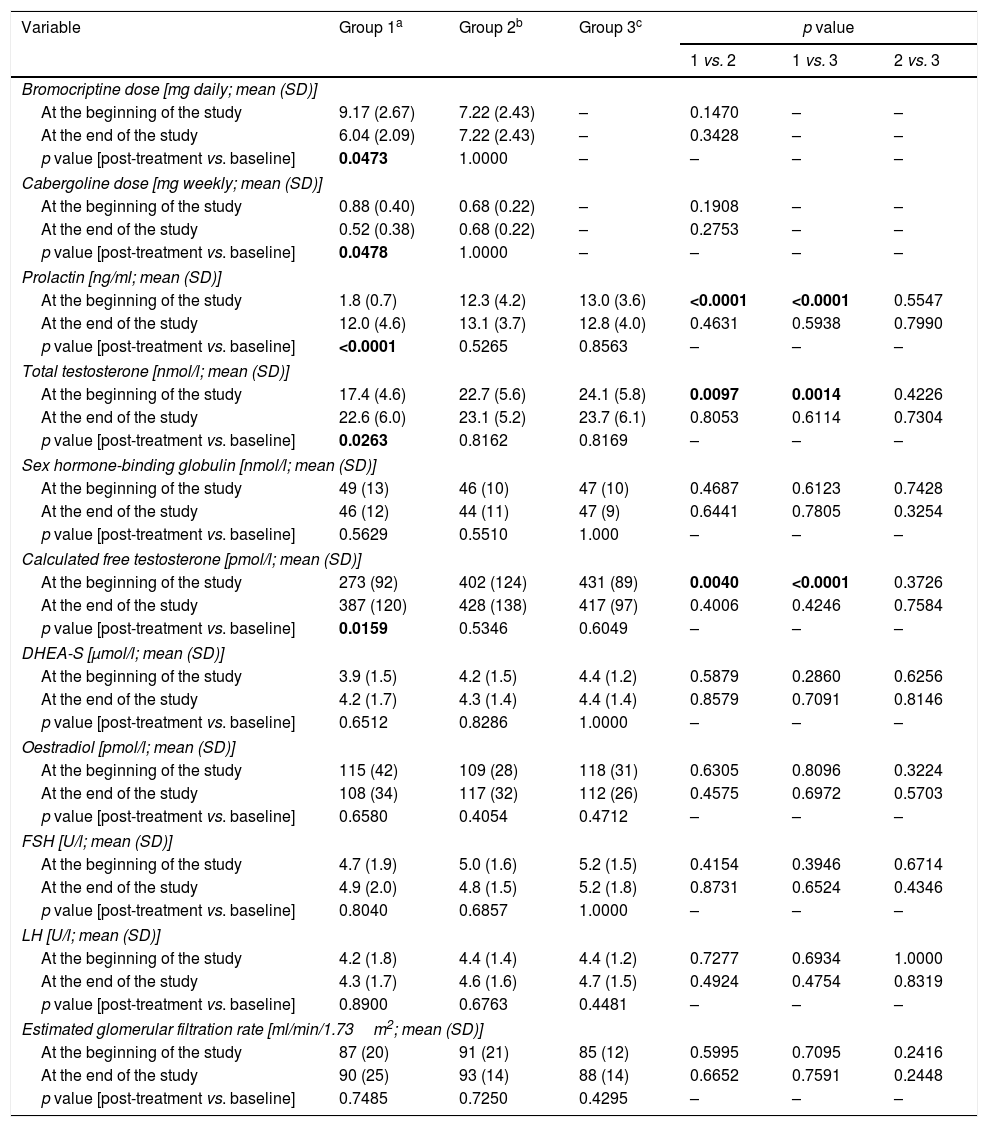
ResultsGeneral characteristics of the study groupsThere were no statistically significant differences between the study arms in age, body mass index, concomitant disorders, smoking, physical activity, education, occupational activity, type of work, number of sexual partners, number and duration of marriages, stress exposure or blood pressure (Table 1). Doses of dopaminergic agents in groups 1 and 2 were similar at baseline and at the end of the study (Table 2).
Sociodemographic characteristics of the study population.
| Variable | Group 1a | Group 2b | Group 3c | p value | ||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||
| Number of patients | 12 | 20 | 24 | – | – | – |
| Age [years; mean (SD)] | 47 (12) | 46 (10) | 44 (11) | 0.8011 | 0.4592 | 0.5350 |
| Body mass index [kg/m2; mean (SD)] | 27.5 (5.0) | 27.1 (4.6) | 26.8 (4.3) | 0.8403 | 0.6654 | 0.8244 |
| Empty sella syndrome/traumatic brain injury/infertility/idiopathic hyperprolactinaemia [%] | 25/25/42/8 | 20/20/50/10 | 25/29/46/0 | 0.6853 | 0.5246 | 0.6831 |
| Smokers [%]/number of cigarettes a day [n; mean (SD)]/duration of smoking [years, mean (SD)] | 42/18 (8)/25 (10) | 40/16 (8)/24 (11) | 46/19 (10)/26 (8) | 0.9011 | 0.8036 | 0.6942 |
| Physical activity: total/once a week/several times a week/once a month [%] | 83/42/25/16 | 80/30/30/20 | 88/38/25/25 | 0.8224 | 0.7528 | 0.8082 |
| Primary or vocational/secondary/university education [%] | 16/42/42 | 20/40/40 | 21/42/37 | 0.7422 | 0.6014 | 0.5224 |
| Occupational activity/blue-collar/white-collar/pink-collar workers [%] | 83/42/42/0 | 80/35/35/10 | 88/42/38/8 | 0.8568 | 0.7150 | 0.5234 |
| Number of sexual partners [n; mean (SD)] | 3.8 (1.8) | 3.6 (1.6) | 3.5 (1.4) | 0.7461 | 0.5854 | 0.8261 |
| Number of marriages [n; mean (SD)]/duration of marriages [months; years (SD)] | 1.3 (0.7)/21 (12) | 1.4 (0.7)/20 (8) | 1.3 (0.7)/20 (10) | 0.8275 | 0.9587 | 0.9412 |
| Stress exposure [%, mean (SD)] | 83 | 85 | 88 | – | – | – |
| Systolic blood pressure [mmHg; mean (SD)] | 134 (15) | 132 (12) | 131 (11) | 0.6807 | 0.4997 | 0.7748 |
| Diastolic blood pressure [mmHg; mean (SD)] | 84 (7) | 82 (7) | 82 (6) | 0.4671 | 0.4401 | 1.0000 |
Abbreviations: SD: standard deviation.
Drug doses and clinical chemistry variables in the study population.
| Variable | Group 1a | Group 2b | Group 3c | p value | ||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||
| Bromocriptine dose [mg daily; mean (SD)] | ||||||
| At the beginning of the study | 9.17 (2.67) | 7.22 (2.43) | – | 0.1470 | – | – |
| At the end of the study | 6.04 (2.09) | 7.22 (2.43) | – | 0.3428 | – | – |
| p value [post-treatment vs. baseline] | 0.0473 | 1.0000 | – | – | – | – |
| Cabergoline dose [mg weekly; mean (SD)] | ||||||
| At the beginning of the study | 0.88 (0.40) | 0.68 (0.22) | – | 0.1908 | – | – |
| At the end of the study | 0.52 (0.38) | 0.68 (0.22) | – | 0.2753 | – | – |
| p value [post-treatment vs. baseline] | 0.0478 | 1.0000 | – | – | – | – |
| Prolactin [ng/ml; mean (SD)] | ||||||
| At the beginning of the study | 1.8 (0.7) | 12.3 (4.2) | 13.0 (3.6) | <0.0001 | <0.0001 | 0.5547 |
| At the end of the study | 12.0 (4.6) | 13.1 (3.7) | 12.8 (4.0) | 0.4631 | 0.5938 | 0.7990 |
| p value [post-treatment vs. baseline] | <0.0001 | 0.5265 | 0.8563 | – | – | – |
| Total testosterone [nmol/l; mean (SD)] | ||||||
| At the beginning of the study | 17.4 (4.6) | 22.7 (5.6) | 24.1 (5.8) | 0.0097 | 0.0014 | 0.4226 |
| At the end of the study | 22.6 (6.0) | 23.1 (5.2) | 23.7 (6.1) | 0.8053 | 0.6114 | 0.7304 |
| p value [post-treatment vs. baseline] | 0.0263 | 0.8162 | 0.8169 | – | – | – |
| Sex hormone-binding globulin [nmol/l; mean (SD)] | ||||||
| At the beginning of the study | 49 (13) | 46 (10) | 47 (10) | 0.4687 | 0.6123 | 0.7428 |
| At the end of the study | 46 (12) | 44 (11) | 47 (9) | 0.6441 | 0.7805 | 0.3254 |
| p value [post-treatment vs. baseline] | 0.5629 | 0.5510 | 1.000 | – | – | – |
| Calculated free testosterone [pmol/l; mean (SD)] | ||||||
| At the beginning of the study | 273 (92) | 402 (124) | 431 (89) | 0.0040 | <0.0001 | 0.3726 |
| At the end of the study | 387 (120) | 428 (138) | 417 (97) | 0.4006 | 0.4246 | 0.7584 |
| p value [post-treatment vs. baseline] | 0.0159 | 0.5346 | 0.6049 | – | – | – |
| DHEA-S [μmol/l; mean (SD)] | ||||||
| At the beginning of the study | 3.9 (1.5) | 4.2 (1.5) | 4.4 (1.2) | 0.5879 | 0.2860 | 0.6256 |
| At the end of the study | 4.2 (1.7) | 4.3 (1.4) | 4.4 (1.4) | 0.8579 | 0.7091 | 0.8146 |
| p value [post-treatment vs. baseline] | 0.6512 | 0.8286 | 1.0000 | – | – | – |
| Oestradiol [pmol/l; mean (SD)] | ||||||
| At the beginning of the study | 115 (42) | 109 (28) | 118 (31) | 0.6305 | 0.8096 | 0.3224 |
| At the end of the study | 108 (34) | 117 (32) | 112 (26) | 0.4575 | 0.6972 | 0.5703 |
| p value [post-treatment vs. baseline] | 0.6580 | 0.4054 | 0.4712 | – | – | – |
| FSH [U/l; mean (SD)] | ||||||
| At the beginning of the study | 4.7 (1.9) | 5.0 (1.6) | 5.2 (1.5) | 0.4154 | 0.3946 | 0.6714 |
| At the end of the study | 4.9 (2.0) | 4.8 (1.5) | 5.2 (1.8) | 0.8731 | 0.6524 | 0.4346 |
| p value [post-treatment vs. baseline] | 0.8040 | 0.6857 | 1.0000 | – | – | – |
| LH [U/l; mean (SD)] | ||||||
| At the beginning of the study | 4.2 (1.8) | 4.4 (1.4) | 4.4 (1.2) | 0.7277 | 0.6934 | 1.0000 |
| At the end of the study | 4.3 (1.7) | 4.6 (1.6) | 4.7 (1.5) | 0.4924 | 0.4754 | 0.8319 |
| p value [post-treatment vs. baseline] | 0.8900 | 0.6763 | 0.4481 | – | – | – |
| Estimated glomerular filtration rate [ml/min/1.73m2; mean (SD)] | ||||||
| At the beginning of the study | 87 (20) | 91 (21) | 85 (12) | 0.5995 | 0.7095 | 0.2416 |
| At the end of the study | 90 (25) | 93 (14) | 88 (14) | 0.6652 | 0.7591 | 0.2448 |
| p value [post-treatment vs. baseline] | 0.7485 | 0.7250 | 0.4295 | – | – | – |
Statistically significant results are marked in bold.
Abbreviations: DHEA-S: dehydroepiandrosterone-sulphate; FSH: follicle-stimulating hormone; IU: international unit; LH: luteinising hormone; SD: standard deviation; SHBG: sex hormone-binding globulin.
Group 1 was characterised by the lowest levels of prolactin (p<0.001 vs. groups 2 and 3), total testosterone (p=0.0097 vs. group 2; p=0.0014 vs. group 3) and calculated free testosterone (p=0.0040 vs. group 2; p<0.0001 vs. group 3). No differences in prolactin, total testosterone or calculated free testosterone were observed between groups 2 and 3. Circulating levels of FSH, LH, DHEA-S and oestradiol did not differ between the study arms.
Reduction of dopamine agonist doses increased levels of prolactin (p<0.0001), total testosterone (p=0.0263) and calculated free testosterone (p=0.0159). After six months, the study groups did not differ in hormone levels.
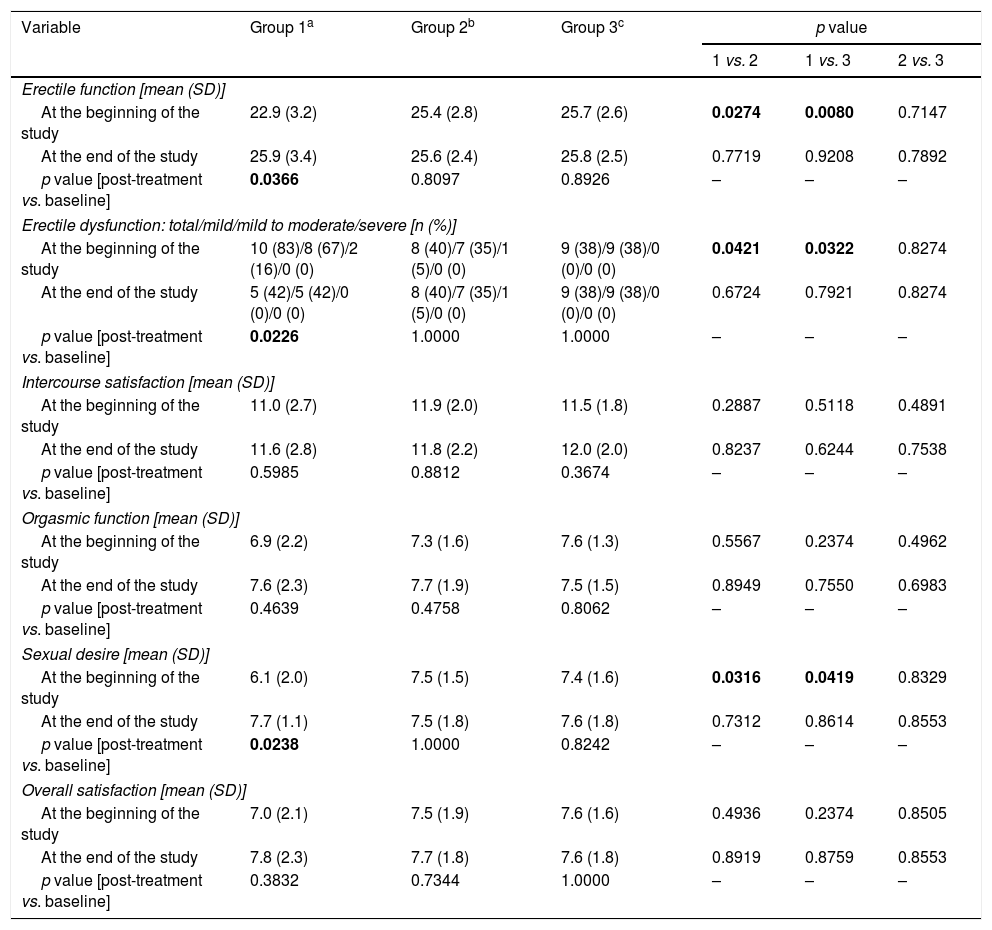
Assessment of sexual function (Table 3)Group 1 differed from groups 2 and 3 in domain scores for erectile function (p=0.0274 vs. group 2; p=0.0080 vs. group 3) and sexual desire (p=0.0316 vs. group 2; p=0.0419 vs. group 3), as well as in percentage of men with erectile dysfunction and severity thereof (p=0.0421 vs. group 2; p=0.0322 vs. group 3). Mild-to-moderate and mild erectile dysfunction were observed in two (16%) and eight (67%) subjects in group 1, as well as in one (5%) and seven (35%) subjects in group 2, respectively. In group 3, nine patients (28%) had mild erectile dysfunction.
Sexual functioning in the study population.
| Variable | Group 1a | Group 2b | Group 3c | p value | ||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||
| Erectile function [mean (SD)] | ||||||
| At the beginning of the study | 22.9 (3.2) | 25.4 (2.8) | 25.7 (2.6) | 0.0274 | 0.0080 | 0.7147 |
| At the end of the study | 25.9 (3.4) | 25.6 (2.4) | 25.8 (2.5) | 0.7719 | 0.9208 | 0.7892 |
| p value [post-treatment vs. baseline] | 0.0366 | 0.8097 | 0.8926 | – | – | – |
| Erectile dysfunction: total/mild/mild to moderate/severe [n (%)] | ||||||
| At the beginning of the study | 10 (83)/8 (67)/2 (16)/0 (0) | 8 (40)/7 (35)/1 (5)/0 (0) | 9 (38)/9 (38)/0 (0)/0 (0) | 0.0421 | 0.0322 | 0.8274 |
| At the end of the study | 5 (42)/5 (42)/0 (0)/0 (0) | 8 (40)/7 (35)/1 (5)/0 (0) | 9 (38)/9 (38)/0 (0)/0 (0) | 0.6724 | 0.7921 | 0.8274 |
| p value [post-treatment vs. baseline] | 0.0226 | 1.0000 | 1.0000 | – | – | – |
| Intercourse satisfaction [mean (SD)] | ||||||
| At the beginning of the study | 11.0 (2.7) | 11.9 (2.0) | 11.5 (1.8) | 0.2887 | 0.5118 | 0.4891 |
| At the end of the study | 11.6 (2.8) | 11.8 (2.2) | 12.0 (2.0) | 0.8237 | 0.6244 | 0.7538 |
| p value [post-treatment vs. baseline] | 0.5985 | 0.8812 | 0.3674 | – | – | – |
| Orgasmic function [mean (SD)] | ||||||
| At the beginning of the study | 6.9 (2.2) | 7.3 (1.6) | 7.6 (1.3) | 0.5567 | 0.2374 | 0.4962 |
| At the end of the study | 7.6 (2.3) | 7.7 (1.9) | 7.5 (1.5) | 0.8949 | 0.7550 | 0.6983 |
| p value [post-treatment vs. baseline] | 0.4639 | 0.4758 | 0.8062 | – | – | – |
| Sexual desire [mean (SD)] | ||||||
| At the beginning of the study | 6.1 (2.0) | 7.5 (1.5) | 7.4 (1.6) | 0.0316 | 0.0419 | 0.8329 |
| At the end of the study | 7.7 (1.1) | 7.5 (1.8) | 7.6 (1.8) | 0.7312 | 0.8614 | 0.8553 |
| p value [post-treatment vs. baseline] | 0.0238 | 1.0000 | 0.8242 | – | – | – |
| Overall satisfaction [mean (SD)] | ||||||
| At the beginning of the study | 7.0 (2.1) | 7.5 (1.9) | 7.6 (1.6) | 0.4936 | 0.2374 | 0.8505 |
| At the end of the study | 7.8 (2.3) | 7.7 (1.8) | 7.6 (1.8) | 0.8919 | 0.8759 | 0.8553 |
| p value [post-treatment vs. baseline] | 0.3832 | 0.7344 | 1.0000 | – | – | – |
Statistically significant results are marked in bold.
Abbreviations: SD: standard deviation
Reduction of dopamine agonist doses increased domain scores for erectile function (p=0.0366) and sexual desire (p=0.0238), as well as in percentage of patients with erectile dysfunction (p=0.0226). No changes in domain scores or percentage of patients with erectile dysfunction were reported in the other two groups. After six months, the study groups did not differ in sexual functioning.
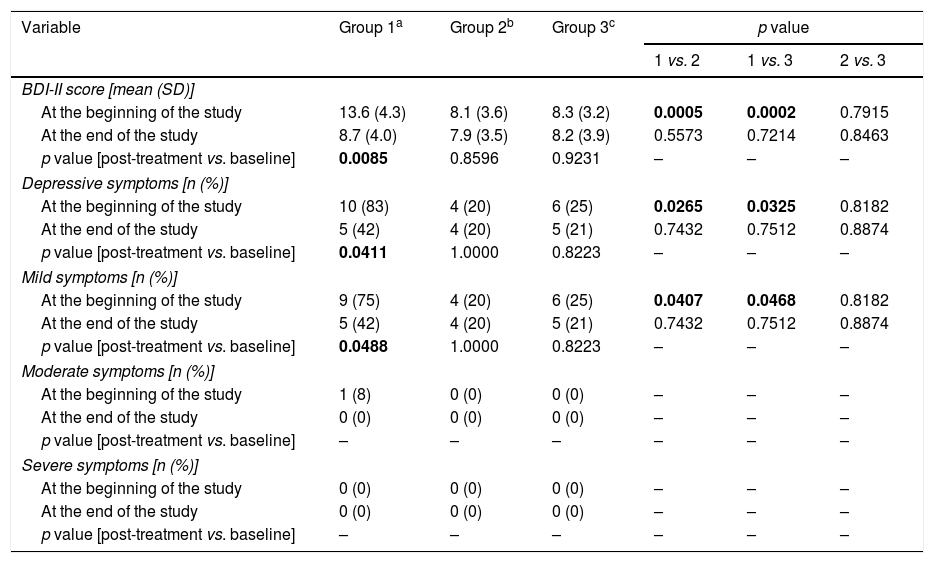
Assessment of depressive symptoms (Table 4)Moderate or severe depressive symptoms were not observed in the study population. Group 1 differed from the other study arms in overall BDI-II score (p=0.0005 vs. group 2; p=0.0002 vs. group 3), as well as in percentage of men with total (p=0.0265 vs. group 2; p=0.0325 vs. group 3) or mild (p=0.0407 vs. group 2; p=0.0468 vs. group 3) depressive symptoms. No differences in BDI-II score or percentage of men with depressive symptoms were observed between groups 2 and 3.
Depressive symptoms in the study population.
| Variable | Group 1a | Group 2b | Group 3c | p value | ||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||
| BDI-II score [mean (SD)] | ||||||
| At the beginning of the study | 13.6 (4.3) | 8.1 (3.6) | 8.3 (3.2) | 0.0005 | 0.0002 | 0.7915 |
| At the end of the study | 8.7 (4.0) | 7.9 (3.5) | 8.2 (3.9) | 0.5573 | 0.7214 | 0.8463 |
| p value [post-treatment vs. baseline] | 0.0085 | 0.8596 | 0.9231 | – | – | – |
| Depressive symptoms [n (%)] | ||||||
| At the beginning of the study | 10 (83) | 4 (20) | 6 (25) | 0.0265 | 0.0325 | 0.8182 |
| At the end of the study | 5 (42) | 4 (20) | 5 (21) | 0.7432 | 0.7512 | 0.8874 |
| p value [post-treatment vs. baseline] | 0.0411 | 1.0000 | 0.8223 | – | – | – |
| Mild symptoms [n (%)] | ||||||
| At the beginning of the study | 9 (75) | 4 (20) | 6 (25) | 0.0407 | 0.0468 | 0.8182 |
| At the end of the study | 5 (42) | 4 (20) | 5 (21) | 0.7432 | 0.7512 | 0.8874 |
| p value [post-treatment vs. baseline] | 0.0488 | 1.0000 | 0.8223 | – | – | – |
| Moderate symptoms [n (%)] | ||||||
| At the beginning of the study | 1 (8) | 0 (0) | 0 (0) | – | – | – |
| At the end of the study | 0 (0) | 0 (0) | 0 (0) | – | – | – |
| p value [post-treatment vs. baseline] | – | – | – | – | – | – |
| Severe symptoms [n (%)] | ||||||
| At the beginning of the study | 0 (0) | 0 (0) | 0 (0) | – | – | – |
| At the end of the study | 0 (0) | 0 (0) | 0 (0) | – | – | – |
| p value [post-treatment vs. baseline] | – | – | – | – | – | – |
Statistically significant results are marked in bold.
Abbreviations: BDI-II: Beck Depression Inventory-Second Edition; SD: standard deviation.
Reduction of dopamine agonist doses decreased overall BDI-II scores (p=0.0085) and led to a decrease in the percentage of subjects with total (p=0.0411) or mild (p=0.0488) depressive symptoms. After six months, overall BDI-II scores and percentages of subjects with total or mild depressive symptoms were similar in all study arms.
CorrelationsAt the start of the study, the overall BDI-II score correlated with domain scores for erectile function, intercourse satisfaction, orgasmic function, sexual desire and overall satisfaction (r values between −0.24 [p<0.05] and −0.48 [p<0.001]). There were correlations between all domain scores of sexual functioning and total prolactin (r values between 0.26 [p<0.05] and 0.41 [p<0.001]), total testosterone (r values between 0.32 [p<0.05] and 0.48 [p<0.001]) and calculated free testosterone (r values between 0.31 [p<0.05] and 0.50 [p<0.001]). The overall BDI-II score also correlated with physical inactivity, stress exposure, systolic blood pressure and diastolic blood pressure (r values between 0.23 [p<0.05] and 0.37 [p<0.01]). In group 1, the impact of reduction of dopamine agonist drugs on domain scores for erectile function and sexual desire correlated with the increase in prolactin levels (erectile function: r=0.38 [p<0.01]; sexual desire: r=0.48 [p<0.001]), the increase in total testosterone (erectile function: r=0.34 [p<0.05]; sexual desire: r=0.40 [p<0.001]), the increase in calculated free testosterone (erectile function: r=0.37 [p<0.01]; sexual desire: r=0.44 [p<0.001]) and the decrease in BDI-II score (erectile function: r=0.32 [p<0.01]; sexual desire: r=0.37 [p<0.01). There were also correlations between baseline prolactin and total testosterone (r values between 0.24 [p<0.05] and 0.31 [p<0.05]) and calculated free testosterone (r values between 0.23 [p<0.05] and 0.37 [p<0.01]), as well as, in group 1, between the drug dose reduction-induced increase in prolactin levels and the changes in total testosterone (r=0.40 [p<0.001]) and in calculated free testosterone (r=0.43 [p<0.001]). No other correlations were significant.
DiscussionIn this study, we enrolled patients treated with two different dopamine agonists for two reasons. First, hypoprolactinaemia in men is very rarely diagnosed22,23 and the study design applied increased the number of subjects participating in our study. Second, treatment with one of two drugs minimised the possible association between the results obtained and the specific properties of only one of them. The study has shown for the first time that very low prolactin levels disturb sexual desire and erection as well as mildly impair wellbeing in young and middle-aged men. These findings cannot be explained by the sociodemographic characteristics of the study population, which were similar in all study arms. Given the exclusion criteria, they did not result from taking other drugs. Finally, they also cannot be attributed to the specific properties of dopaminergic agents because sexual dysfunction and depressive symptoms did not occur more frequently in subjects suitably treated with these drugs than in dopamine agonist-naïve men with prolactin levels within the reference range. They are much more likely to be associated with subnormal prolactin production. In line with this explanation, frequencies of sexual and mood disturbances were inversely correlated with prolactin levels and were the same as in the control group after normalisation of prolactin levels, while improvements in libido and erection were correlated with the effect of reduction of drug doses on prolactin levels. The results obtained indicate that hypoprolactinaemia cannot be regarded as a benign condition, in line with previous observations showing that very low prolactin levels in men are associated with an increased risk of premature ejaculation, oligozoospermia, asthenospermia, impaired seminal vesicle function, impaired penal blood flow, hypoandrogenism, metabolic syndrome and anxiety.22,23 Taking into account the absence of between-group or intra-group differences in glomerular filtration rate, it seems that sexual dysfunction and abnormal wellbeing resulted from the inhibitory effect of both drugs on lactotroph function. On the basis of the results obtained, two conclusions may be drawn. First, all men with concomitant erectile dysfunction and hypolibido of unknown origin should be evaluated for hypoprolactinaemia. Secondly, hypoprolactinaemia-induced sexual dysfunction and depressive symptoms in men are not permanent and resolve after normalisation of prolactin levels.
The impact of prolactin deficiency on libido and desire was in part secondary to the decrease in free testosterone levels. In line with this explanation, prolactin levels correlated with both total testosterone levels and calculated free testosterone levels. The latter parameter reflects the unbound fraction of this hormone and, if calculated using Vermeulen's formula, correlates well with free testosterone assessed by equilibrium dialysis.16,17 Moreover, a dopamine agonist dose reduction-induced increase in prolactin levels was found to be associated with changes in total and calculated free testosterone. The notion of a relationship between low prolactin levels and reduced testosterone levels is supported by the results of previous studies. Bromocriptine-induced hypoprolactinaemia led to a decrease in testosterone levels and attenuated testosterone response to stimulation with chorionic gonadotropin,24 while subnormal testosterone levels were reported in two-thirds of patients with low prolactin levels.22 It is possible that hypoprolactinaemia-induced changes in testosterone levels are associated with a modulatory effect of prolactin on testicular gonadotropin action. Normal levels of prolactin were found to enhance the impact of LH on Leydig cells, but this effect may be absent if prolactin levels are low. It has been found that animals with lactotroph hypofunction induced by dopamine agonist treatment had decreased numbers of LH receptors, but this effect was absent if the animals were given prolactin injections.25 Interestingly, our population was characterised by relatively high mean body mass index values, suggesting insulin resistance in many participants. Considering that insulin resistance is often accompanied by impaired testosterone production,26 this finding may explain why a significant proportion of dopamine agonist-naïve subjects with prolactin levels within the reference range had relatively low IIEF-15 domain scores. The other hormones investigated did not seem to mediate the impact of lactotroph hypofunction on sexual function. Circulating levels of oestradiol, DHEA-S and gonadotropins were similar in each study arm, stayed at similar levels throughout the study period and did not correlate with any domain score.
It is difficult to explain the discrepancy between our results and the hypersexuality observed by other authors.11–14 Our study's strict inclusion and exclusion criteria meant that our study population was more homogeneous, and the results obtained seem to have been less affected by the impact of concomitant disorders and concurrent therapies than the results of other authors. In addition, the participants in this study were treated with D2 dopamine receptor agonists, while hypersexuality and other impulse control disorders have been reported mainly following high doses of pramipexole and ropinirole, which have preferential affinity for the D3 receptor.11 Furthermore, previous studies have concentrated mainly on symptoms reported by patients, while this study investigated all relevant domains of male sexual functioning (erectile function, intercourse satisfaction, orgasmic function, sexual desire and overall satisfaction) using a validated inventory with proven high reliability.18 Finally, this study is the only one that has screened patients based on not only dopamine agonist treatment but also prolactin levels.
Although IIEF-15 evaluates five significant domains of male sexual function, hypoprolactinaemic men differed from the remaining two groups only in desire and erectile function. This means that these two domains are more susceptible to lactotroph hypofunction and/or feedback changes in dopamine transmission in the tuberoinfundibular pathway than orgasmic function, intercourse satisfaction and overall satisfaction. Interestingly, degree of sexual dysfunction was found to depend on severity of hypothyroidism and was more severe in overt than subclinical thyroid hypofunction.19 Although extensively demonstrated, the role of testosterone in the regulation of sexual functioning is modified by the impact of organic, relational and psychological factors.27 Taking into account all these complicated reciprocal relationships, the impact of hypoprolactinaemia as a relatively mild form of dyshormonogenesis may be less obvious than that observed in men with severe endocrine disturbances.
The presence of correlations between depressive symptoms and lower domain scores for libido and erectile functioning at baseline, as well as of similar relationships between dose reduction-induced changes in these variables, support the existence of relationships between them. It is difficult, however, to conclude whether sexual disturbances together with other factors correlate with BDI-II scores and whether physical inactivity, stress exposure and blood pressure contribute to the increased prevalence of depressive symptoms in hypoprolactinaemic patients or impaired wellbeing is a factor disturbing sexual functioning. Our results are consistent with the results of a previous study that enrolled women with seasonal affective disorder who were characterised by lower prolactin levels compared to healthy controls.28 It is possible that the link between hypoprolactinaemia and impaired mood is associated with the interactions between prolactin release and the monoamine neurotransmitter systems that play a role in the development of depression29 or with the lack of a modulatory effect of prolactin on the immune response and inflammatory processes in the limbic system.30
This study had several shortcomings which limited the conclusions that could be drawn. The most significant study limitations were a small sample size and a short observation period. Like other self-administered questionnaires, the IIEF-15 and BDI-II scoring systems are subjective in nature. We cannot rule out the possibility that the effect of hypoprolactinaemia on sexual functioning and depressive symptoms is different in elderly men. Finally, we cannot unequivocally answer the question of whether the impact on sexual function and mood is similar in hypoprolactinaemia that is not drug-induced.
In conclusion, dopamine agonist-induced hypoprolactinaemia impaired sexual desire and erectile function and led to reduced wellbeing in young and middle-aged men. The degree of sexual dysfunction correlated with the degree of prolactin deficiency and testosterone production. Following normalisation of prolactin levels, the frequency of these disturbances did not differ from that observed in dopamine agonist-naïve normoprolactinaemic men. Our results suggest that sexual dysfunction and depressive symptoms in men are a consequence of lactotroph hypofunction.
Funding informationThis study was not supported by any external source of funding.
Authors’ contributionR.K.: concept and design, data analysis and interpretation, drafting of the paper; K.K.: data analysis and interpretation; B.O.: concept and design, critical review of the manuscript for intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the study.
Conflicts of interestThe authors declare they have no conflicts of interest.