Hyperprolactinemia may be due to physiological or pathological causes, and may be asymptomatic or induce hypogonadism, infertility, and/or galactorrhea. It is important to take prolactin samples while avoiding stress, as this may increase prolactin levels. Therefore, our aim was to assess the value of prolactin serial sampling after brachial vein cannulation.
Patients and methodsSixty-six patients (34.9±11.8 years of age, 92.4% female) with an initial elevated random prolactin level were included. A prolactin sample was drawn at baseline and after a 30min rest.
ResultsThe median referral prolactin level was 37.4ng/ml (interquartile range [IQR* 23.3), the baseline prolactin level at serial sampling was 19.5ng/ml (IQR 8), and the value after a 30min rest was 17.1ng/ml (IQR 7.9). Hyperprolactinemia was not confirmed by serial sampling in 45 patients (68.2%). There were no statistically significant differences in referral prolactin levels between patients with and without confirmed hyperprolactinemia (41.2ng/ml and 36.7ng/ml respectively, p=0.3). Galactorrhea was found in 13.6% of patients, amenorrhea or oligomenorrhea in 28.8%, infertility in 7.6%, erectile dysfunction in 4.6%, and gynecomastia in 3%, while 45.5% were asymptomatic. There were no statistical differences regarding the presence or absence of any of these symptoms and subsequent confirmed hyperprolactinemia. Fifty-seven patients (86.4%) were discharged after the results of the prolactin serial sampling were obtained.
ConclusionsProlactin serial sampling may be a useful test to detect artefactual hyperprolactinemias, thus avoiding unnecessary additional tests and treatments.
La hiperprolactinemia puede ser debida a causas fisiológicas o patológicas, y puede ser asintomática o inducir hipogonadismo, infertilidad y/o galactorrea. Es importante obtener las muestras de prolactina evitando situaciones de estrés, puesto que este puede incrementar sus niveles. Por tanto, nuestro objetivo era evaluar la utilidad de la realización de curvas de prolactina mediante canalización de la vena braquial.
Materiales y métodosSe incluyeron 66 pacientes (edad: 34,9±11,8 años; 92,4% mujeres) con una prolactina aleatoria inicial elevada. Se obtuvieron una muestra de prolactina basal y otra tras un reposo de 30min.
ResultadosLa prolactina mediana inicial fue 37,4ng/ml (IQR: 23,3), la prolactina basal de la curva 19,5ng/ml (IQR: 8), y tras 30min de reposo, 17,1ng/ml (IQR: 7,9). La curva descartó una hiperprolactinemia en 45 pacientes (68,2%) No hubo diferencias estadísticamente significativas en la prolactina de derivación entre aquellos pacientes en los que se confirmó una hiperprolactinemia y aquellos que no (41,2 vs. 36,7ng/ml; p=0,3). Un 13,6% de los pacientes presentaron galactorrea, un 28,8% amenorrea u oligomenorrea, un 7,6% infertilidad, un 4,6% disfunción eréctil y un 3% ginecomastia. El 45,5% estaban asintomáticos. No hubo diferencias estadísticamente significativas entre la presencia o ausencia de ninguno de estos síntomas y una hiperprolactinemia confirmada posteriormente. Se pudo dar de alta a 57 pacientes (86,4%) tras la obtención de los resultados de la curva de prolactina.
ConclusionesLa curva de prolactina puede ser una prueba útil pata detectar falsas hiperprolactinemias, evitando la realización de pruebas complementarias y tratamientos adicionales innecesarios.
Prolactin is a polypeptide of 199 aminoacids which is mainly secreted by the lactotroph cells in the pituitary gland.1 Its synthesis and secretion is influenced by many factors, being hypothalamic dopamine one of the keystones, as it suppresses both. However, other factors such as oestrogen, thyrotropin-releasing hormone, fibroblast growth factor, vasoactive intestinal peptide, serotonin and epidermal growth factor, on the contrary, stimulate prolactin synthesis and secretion.1
It is also important to mention that prolactin is secreted in a pulsatile manner, with up to 14 peaks per-24h2, and with a circadian variation, reaching its maximum concentration at night.3
Prolactin's main function in humans is to induce lactation, and it is therefore physiologically elevated during pregnancy and breastfeeding, as well as during exercise, sleep or under stress. However, elevated levels may also be found under systemic disorders, such as renal failure, cirrhosis, hypothyroidism or polycystic ovary syndrome.4 Other pathological aetiologies of hyperprolactinemia include hypothalamic or pituitary pathology (being prolactinoma the most relevant), or drugs that interfere in the production, transport or action of dopamine, such as neuroleptics/antipsychotic agents, antidepressants or oestrogens, amongst others.5
Regardless of the cause of hyperprolactinemia, it may produce hypogonadism, infertility, and galactorrhoea, although some individuals remain asymptomatic.5 Symptomatology may influence the treatment selected for each particular patient.
As we can observe, many factors can influence prolactin levels, and therefore establishing the diagnosis of hyperprolactinaemia may not be as easy as thought at first glance, even though many data can be obtained with a thorough clinical history. In fact, in our clinical practice, referral to the Endocrinology Department due to hyperprolactinaemia is very common. Even when interfering medications, comorbidities or physiological situations have been ruled out (for example, discontinuing the drugs for three days, as recommended by the Endocrine Society Guidelines), the time of the day or the conditions in which blood sampling takes place may influence the prolactin values that we obtain.5 However, the Endocrine Society Guidelines for the diagnosis and treatment of hyperprolactinemia advise that a single measurement of prolactin in a blood sample obtained at any time of the day will usually be adequate to document hyperprolactinaemia, as long as the sample is withdrawn without excessive venepuncture stress.5,6 Nonetheless, a stressful venepuncture is subjective and may be difficult to determine, and this is the reason why in our media it is common to obtain prolactin after serial blood sampling in those patients which showed an initial elevated prolactin level. However, little has been published about the utility of prolactin serial blood sampling in order to rule out artefactual hyperprolactinaemias secondary to the stress of venepuncture, and data are controversial.7–9 We therefore aimed to analyse the usefulness of this procedure according to the data collected in our Department.
Materials and methodsAn observational retrospective analysis was carried out, which included all of the patients referred to our outpatient clinic due to hyperprolactinaemia in which a prolactin serial sampling was done between 2015 and 2017. Hyperprolactinaemia was defined as a prolactin above our laboratory's reference range (>18.6ng/ml for men and women). Exclusion criteria included pregnancy, breastfeeding, a non-treated overt hypothyroidism, having received treatment with dopamine agonists or being under medications or drugs that could interfere in prolactin synthesis, action or transport.
Serial blood sampling was carried out with the patient in a sitting position in a quiet room. In the first place, a catheter was inserted into a brachial vein, and a prolactin was obtained at baseline and after 30min rest. The duration of prolactin's half-life is controversial, and in the literature it has been described between 10 and 50min.10,11 In order to avoid venepuncture stress, if prolactin's half-life was considered to be 10min, the more separated the samples, the lower the values of prolactin (30min would correspond to 3 half-lives). However, some authors describe prolactin's half-life as being longer. If this was considered to be the case, a 30min interval would be more accurate than a 20min one.
Patients were not prevented from eating during the previous hours to the sampling. However, they were recommended to attend their appointment having rested correctly and avoiding stressful situations, as well as not having smoked or drunk alcohol since the night before. No specific indications were given to pre-menopausal women about timing of the sampling according to their menstrual cycle.
All of the referral prolactin values were measured in the same laboratory as the serial sampling prolactin values, with an immunometric immunoassay technique (VITROS 5600, Ortho Clinical Diagnostics). It involves the simultaneous reaction of prolactin present in the sample with a biotinylated antibody (sheep polyclonal anti-prolactin) and a horseradish peroxidase (HRP)-labelled antibody conjugate (mouse monoclonal anti-prolactin). The antigen–antibody complex is captured by streptavidin on the wells and unbound materials are removed by washing. Following this, the bound HRP conjugate is measured by a luminescent reaction in which a reagent containing luminogenic substrates (a luminol derivative and a peracid salt) and an electron transfer agent are added to the wells. The HRP in the bound conjugate catalyses the oxidation of the luminol derivative, producing light, and the electron transfer agent (a substituted acetanilide) increases the level of light produced and prolongs its emission. Finally, the light signals are read by the system. Therefore, the amount of HRP conjugate bound is directly proportional to the concentration of prolactin present.
When a prolactin below 18.6ng/ml was obtained with serial sampling (either at the baseline or after 30min rest), we considered that the patient did not have a “confirmed hyperprolactinaemia”. However, in clinical practice, very slight elevations of prolactin may not be considered relevant and may not change our diagnostic or therapeutic approach. That is the reason why we also evaluated if patients were discharged after the results of the prolactin serial sampling or not. As it is a retrospective observational analysis, a specific cut-off point for this was not established beforehand.
Additional data such as age, gender or symptoms were obtained by reviewing the patients’ clinical history.
The statistical analysis was carried out with SPSS v22. Descriptive statistics were used to express the mean±standard deviation (SD) for continuous, normally distributed variables. For those variables that were not normally distributed, the median and interquartile ranges (IQR) were obtained.
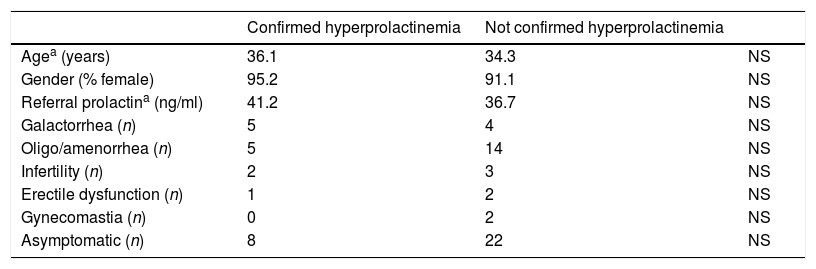
Differences between the group of patients with a confirmed hyperprolactinemia and the group without were compared using chi-squared tests or Fisher's exact test for categorical variables and Mann Whitney U-tests for continuous variables (Table 1).
Comparative of variables between patients with confirmed hyperprolactinemia and those without it.
| Confirmed hyperprolactinemia | Not confirmed hyperprolactinemia | ||
|---|---|---|---|
| Agea (years) | 36.1 | 34.3 | NS |
| Gender (% female) | 95.2 | 91.1 | NS |
| Referral prolactina (ng/ml) | 41.2 | 36.7 | NS |
| Galactorrhea (n) | 5 | 4 | NS |
| Oligo/amenorrhea (n) | 5 | 14 | NS |
| Infertility (n) | 2 | 3 | NS |
| Erectile dysfunction (n) | 1 | 2 | NS |
| Gynecomastia (n) | 0 | 2 | NS |
| Asymptomatic (n) | 8 | 22 | NS |
A simple linear regression was also calculated using the programming language R to test if baseline prolactin could predict 30min serial sampling prolactin.
Finally, the study was approved by the ethics committee.
ResultsIn this study, a total of 66 patients were included, with a mean age of 34.9 years, SD 11.8 (15–70). 92.4% of the sample members were female.
As the obtained prolactin values at referral and at serial sampling did not obey to a normal distribution, the median was taken as the reference value. The results were 37.4ng/ml (IQR 23.3; sample range 20.6–277.6ng/ml) for referral prolactin, 19.5ng/ml (IQR 8) for baseline prolactin at serial sampling, and 17.1ng/ml (IQR 7.9) for 30min serial sampling.
21 of the evaluated patients (31.8%) had a confirmed hyperprolactinaemia. No statistically significant differences in age or gender were observed comparing those patients in which there was a confirmed hyperprolactinaemia and those in which there was not.
There was a tendency to higher prolactin referral values in the group with a posterior confirmed hyperprolactinaemia, but it was not statistically significant (41.2ng/ml vs 36.7ng/ml; p=0.3).
As mentioned above, a simple linear regression was carried out to test if the baseline prolactin significantly predicted the 30min serial sampling prolactin. The results of the regression indicated that the model explained 54.8% of the variance and that it was significant, F(1,64)=77.6, p<0.001. Baseline prolactin was found to significantly predict the 30min serial sampling prolactin (β1=0.953, p<0.001). The final predictive model was: 30min serial sampling prolactin=1.144 + 0.953 * baseline prolactin.
Of the patients included in our study, 13.6% presented with galactorrhoea, 28.8% with amenorrhoea or oligomenorrhoea, 7.6% with infertility (one patient combined galactorrhoea, irregular menses and infertility), 4.6% with erectile dysfunction and 3% with gynecomastia. It is noticeable however, that in 45.5% of the patients referred to our Department, no symptoms were present when the first value of elevated prolactin was obtained. These data were analysed according to Chi Square and Fisher's exact test, showing no statistical differences between the presence or absence of any of these symptoms and posterior confirmed hyperprolactinaemia.
As mentioned before, in clinical practice, very slight elevations of prolactin are usually interpreted as not having any pathological significance and, therefore, no further diagnostic tests are performed, and/or no treatment is established. As this was an observational analysis, we had not established a clear cut-off point to decide which elevations of prolactin were significant and which were not. However, we did register in which cases further evaluation/follow-up took place or if on the contrary, the patient was discharged. Out of the 66 patients that were evaluated, 57 (86.4%) were discharged after obtaining the results of prolactin serial sampling.
DiscussionWe performed a retrospective observational study to evaluate the frequency of confirmed hyperprolactinaemia after conducting prolactin serial sampling in patients referred to our outpatient clinic due to hyperprolactinaemia. As it can be observed in the results presented above, only 31.8% of the patients with an initial elevated prolactin in which serial sampling was carried out, were shown to have a confirmed hyperprolactinaemia. Therefore, 68.2% of the initial elevated prolactins were not true hyperprolactinaemias. These results would encourage using prolactin serial sampling in order to avoid misdiagnosis (some studies have suggested that up to 10% of the general population may present with an incidental pituitary adenoma on imaging12) and could enable the medical team to avoid unnecessary additional tests and/or treatments in many patients.
However, as mentioned before, little has been published on prolactin serial sampling with vein cannulation, and results are heterogeneous. Whyte et al. carried out a study with 235 patients in which after an elevated referral prolactin, a new determination was carried out with vein cannulation, obtaining a baseline prolactin, and another sample after 120min rest. In 17% of cases the baseline cannulated prolactin was normal, and in 9%, normal values were obtained after rest.9 The difference between the results obtained in our study and the one carried out by Whyte et al. is remarkable, as in the latter, subjects required at least one of the following indications for a serial prolactin sampling: borderline raised referral prolactin (24–28.3ng/ml); no clinical features of hyperprolactinaemia; clinical suspicion of stress-induced hyperprolactinaemia. Under these circumstances, one would have expected to obtain a higher proportion of artefactual hyperprolactinaemias compared to our study, in which carrying out serial sampling was not dependent on the levels of the initial referral prolactin or on the absence of symptoms.
Muneyyirci-Delale et al. also obtained similar results to Whyte et al. In this case, patients were referred for serial sampling after two random samples in which prolactin was elevated. After vein cannulation, 3–6 samples were obtained at 15–30min intervals. 28% of the patients were shown to be euprolactinemic by the serial sampling.7
In another study performed by Briet et al., no artefactual hyperprolactinaemias were detected after carrying out prolactin serial sampling. This could be justified by the fact that in this case the resting period was only 15min, and therefore, it could be argued that it was not long enough to avoid the effect of pulsatility in the secretion of prolactin.2,8
As it can be appreciated in our results, a large proportion of artefactual hyperprolactinaemias (68.2%) was detected with serial sampling in our media. It is also important to perceive that this proportion of artefactual hyperprolactinaemias was detected with only two prolactin determinations, in comparison with Muneyyirci-Delale et al.’s study, where several determinations per patient took place. Less time investment was also needed, as in Muneyyirci-Delale et al.’s study several determinations every 15–30min were carried out, and in Whyte et al.’s study, the second determination was obtained 120min after the baseline prolactin.7,9 On the contrary, in our study, serial sampling included basal prolactin and 30min after rest. It could therefore be concluded that in our media prolactin serial sampling seems to be a cost-effective test, which detects a large proportion of artefactual hyperprolactinaemias, faster and in a more economical way.
Nevertheless, it must be noted that in our sample, there was a great difference between median referral prolactin and median baseline prolactin in serial sampling (37.4ng/ml vs 19.5ng/ml). This could raise the question of whether the second determination of the serial sampling could be avoided, as conditions in which blood sampling was carried out seem to have had a great impact on the results. However, if only baseline prolactin was measured, according to the linear regression model that was calculated, 12 patients would have been misdiagnosed as having a hyperprolactinemia when they did not.
Another aspect to consider is whether there is a valid threshold above which serial prolactin sampling should not be considered. At Whyte et al.’s study, a ROC curve was constructed to establish possible cut-off values for “accepting” a single referral prolactin, and they established 96.2ng/ml (×4 upper range of normal) had 97% specificity to detect true hyperprolactinaemia in women.9 However, according to our results, prolactin values in both groups (with and without confirmed hyperprolactinaemia) overlapped, and we were not able to recommend a cut-off point. In fact, on the group that did not have confirmed elevated prolactin after serial sampling, values as high as 277.6ng/ml were reported.
In our cohort, the normalisation of prolactin in the serial sampling was not associated with the presence or absence of symptoms. At Whyte et al.’s study, overall clinical symptoms were not a reliable guide either, and galactorrhoea was the only symptom that was significantly more common amongst patients with true hyperprolactinaemia.9 On Muneyyirci-Delale et al.’s study all patients included were symptomatic, and therefore this aspect was not evaluated.7
However, it must be kept in mind that there are some limitations to our study, such as the fact that pre-menopausal women were not given any instructions about timing of the sampling according to their menstrual cycle, that it is a retrospective study and that our total sample size was limited, influencing statistical analysis of some of the variables.
ConclusionsProlactin serial sampling with two determinations (at baseline and after 30min) seems to be a useful method to confirm true hyperprolactinaemia. It is a simple procedure which allowed to rule out real hyperprolacinaemia in 68.2% of the patients in our sample. This enables the medical team to avoid unnecessary additional tests and/or treatments in many patients, making medical attention in this context much more efficient.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestAuthors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.