Postprandial hyperinsulinaemic hypoglycaemia is a common complication of bariatric surgery. Although in general its evolution is mild and self-limited, it can lead to neuroglycopaenia and compromise the patient's safety and quality of life. The aim of this document is to offer some recommendations to facilitate the clinical care of these complex patients, reviewing the aetiopathogenesis, its diagnosis and treatment that, sequentially, will include dietary and pharmacological measures and surgery in refractory cases. In the absence of high-quality studies, the diagnostic and therapeutic approach proposed is based on the consensus of experts of the Grupo de Obesidad de la Sociedad Española de Endocrinología y Nutrición [Obesity Group of the Spanish Society of Endocrinology and Nutrition], GOSEEN. Those undergoing bariatric surgery should be informed of the possibility of developing this complication.
La hipoglucemia hiperinsulinémica postprandial es una complicación frecuente de la cirugía bariátrica. Aunque en general tiene una evolución leve y autolimitada, puede cursar con neuroglucopenia y comprometer la seguridad y la calidad de vida del paciente. El objetivo de este documento es ofrecer unas recomendaciones para facilitar la atención clínica a estos pacientes complejos, revisando la etiopatogenia, su diagnóstico y tratamiento que, de manera secuencial, incluirá medidas dietéticas, farmacológicas y cirugía en casos refractarios. Ante la ausencia de estudios de alta calidad, el abordaje diagnóstico y terapéutico propuesto se basa en el consenso de expertos del Grupo de Obesidad de la Sociedad Española de Endocrinología y Nutrición, GOSEEN. Las personas sometidas a cirugía bariátrica deben ser informadas de la posibilidad de desarrollar esta complicación.
Postprandial hypoglycaemia is a complication of bariatric surgery (BS) of unknown aetiology, for which the diagnosis and therapeutic approach are challenging, especially in the most severe cases. Initially described as late dumping syndrome, referring to the accelerated gastric emptying that is one of the pathogenic mechanisms described, today, the term postprandial hyperinsulinaemic hypoglycaemia (PHH) is preferred. However, some authors include only the most severe cases under this heading. Its prevalence in different series varies, depending on the type of surgical technique, severity and diagnostic tool. In general, they appear most frequently after techniques that exclude the pylorus, such as gastric bypass (GBP)1. In a study based on a Swedish national registry, a risk of hospital admission for severe hypoglycaemia or some other condition (epilepsy, confusion, syncope) was observed in people with a history of GBP of 0.2%2, with a prevalence of 0.47% in the multicentre Spanish registry of the Grupo de Obesidad de la Sociedad Española de Endocrinología y Nutrición (GOSEEN) [Obesity Group of the Spanish Society of Endocrinology and Nutrition]3. However, if less severe self-reported hypoglycaemia is included, the prevalence is more variable: between 0.1% after GBP and 0.02% after vertical sleeve gastrectomy (VSG)1, to 30%4 and up to 75% if continuous glucose monitoring (CGM) is used5.
This paper reviews the aetiopathogenesis, diagnostic methods and nutritional, pharmacological and surgical treatment of postprandial hypoglycaemia after BS. GOSEEN has agreed on these recommendations as a group of experts.
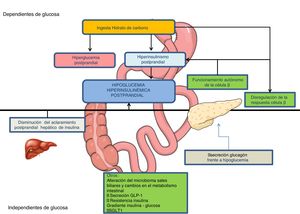
Pathophysiological mechanismsGastrointestinal surgery produces alterations in the regulation of hydrocarbon metabolism, creating conditions that favour PHH6,7 among which the following stand out (Fig. 1):
Postprandial hyperglycaemiaIn GBP, the passage of nutrients to the jejunum is accelerated up to 100 times, and the intestinal mucosa hypertrophy favours a more rapid absorption of glucose. Gastric emptying is also accelerated after VSG6,7.
Postprandial hyperinsulinaemiaLess suppression of insulin secretion in the presence of hypoglycaemia and greater sensitivity of β cells to increased blood glucose levels have been described after GBP7, mediated both by intestinal hormonal factors (increase in GLP-1 secretion)8,9 and by non-hormonal factors6–8 (postprandial elevation of unconjugated bile acids)7, which will be accompanied by an earlier and more intense peak of postprandial insulinaemia.7,8 One of the first hypotheses proposed was the increase in the mass of pancreatic β cells with nesidioblastosis, as a result of the exaggerated secretion of GLP-110. However, a greater mass of β cells, or concentration of GLP-1 receptors at the pancreatic level, or greater sensitivity of pancreatic β cells to GLP-1 have not been demonstrated in affected patients7,10,11. Patients with hypoglycaemia after GBP also present a 30% decrease in postprandial insulin clearance, mainly in the liver10.
Altered glucagon response to hypoglycaemiaMechanism that would perpetuate hypoglycaemia once established7,9.
Other mechanismsThese include, among others, changes in the intestinal microbiota, in the glucose and insulin gradient of the porto-systemic axis, and in the action of GLP-1 on vagal afferents, as well as a reduction in endogenous glucose production12, and an increase in bile salts and intestinal glucose absorption due to increased expression of sodium-glucose cotransporter type 1 (SGLT1)7,13–15.
Definition and clinical evaluationThere is currently no consensus on the plasma glucose concentration that defines hypoglycaemia after bariatric surgery. Although some authors assume the ADA definition (≤70 mg/dl), GOSEEN, as an expert group, as well as the majority of authors, agrees with using thresholds lower than 60 mg/dl or 3.3 mmol/l, especially after provocation tests, or less than 50 mg/dl or 2.8 mmol/l, when it is spontaneous6,16–19.
PHH is diagnosed when low postprandial blood glucose (<50−60 mg/dl) coexists with inappropriately high insulinaemia (>3–5 μU/mL, C-peptide >0.6 ng/mL), with signs and symptoms compatible with hypoglycaemia that improve after correction (Whipple's triad)10,20. Hypoglycaemia causes adrenergic symptoms (palpitations, tachycardia, anxiety, shaking), cholinergic symptoms (sweating, warmth, nausea, hunger) and, less frequently, symptoms of neuroglycopenia (dizziness, weakness, confusion, blurred vision, focal neurological deficit, convulsions and coma).
Fasting blood glucose and insulinaemia are normal in these cases. However, in clinical practice, it is difficult to obtain all these parameters during the acute episode.
It is essential to take a medical history with a detailed description of the symptoms, their severity (frequency, neuroglycopenia), their relationship with fasting, possible triggers and the time elapsed since BS. Severe hypoglycaemia is uncommon and usually appears 1−5 years after surgery. In the event of hypoglycaemia a few months after surgery (less than six months) and/or when fasting, other aetiologies of hypoglycaemia other than PHH must be ruled out, such as nesidioblastosis or insulinoma (Table 1).
Differential diagnosis of hypoglycaemia with postprandial hyperinsulinism.
| Early dumping syndrome | Late dumping syndrome or postprandial hypoglycaemia with hyperinsulinism | Nesidioblastosis | Insulinoma | |
| Baseline | 30−60 min after food Shortly after surgery | 1−3 h after food 1−5 years after surgery | Fasting hypoglycaemia Any time after surgery | Fasting and postprandial hypoglycaemia Any time after surgery |
| Clinical signs | Vasomotor and gastrointestinal symptoms | Adrenergic and cholinergic symptoms Sometimes symptoms of neuroglycopenia of varying severity | Symptoms of severe neuroglycopenia | Symptoms of severe neuroglycopenia |
| Cause | Rapid emptying of hyperosmolar gastric contents into the small intestine | Multifactorial aetiology Increased postprandial secretion of insulin-mediated by GLP-1 | Abnormal proliferation of pancreatic islet cells with diffuse involvement of the pancreas | Tumour with increased pancreatic β-cell mass |
| Treatment | Resolution with dietary modifications | Usually responds to dietary treatment Severe cases may require pharmacological and/or surgical treatment | Does not respond to dietary treatment. Need for medical and/or surgical treatment | Indication for surgical treatment |
| Frequency | 20−30% | 1−6% 0.2−0.3% (severe cases with neuroglycopenia) | Isolated cases in literature | Isolated cases in literature |
Several questionnaires have been developed for the diagnosis of dumping syndrome that are not useful in PHH since they have not been validated in patients with previous BS. The following have been used after BS.
- -
Dumping Severity Score (DSS)21: with this questionnaire, a prevalence of early and late dumping syndrome after GBP of 18.8% and 11.7%, respectively, has been described22.
- -
The Edinburgh Hypoglycaemia Scale assesses symptoms of hypoglycaemia in patients with diabetes; an adaptation has been used for patients that have undergone BS. Up to a third of them reported symptoms compatible with postprandial hypoglycaemia4.
Hypoglycaemia assessment is complex since most methods can detect hypoglycaemia in asymptomatic subjects.
Although capillary blood glucose at the time of symptoms can guide the diagnosis, it is not recommended as a confirmation method due to the lack of precision of the devices in the hypoglycaemia range23. Up to 50% of patients with GBP present with hypoglycaemia at some point, most of them asymptomatic.
Continuous glucose monitoring (CGM) measures interstitial glucose and provides 24-h blood glucose information. However, its reliability in the hypoglycaemic range is limited24. Up to 75% of patients with GBP have hypoglycaemia on CGM5,7.
Using prolonged oral glucose overload is not recommended, since with this test practically half of the patients present blood glucose levels <47 mg/dl25, even when they are asymptomatic13,26 and it is also very poorly tolerated by these patients.
The Mixed Meal Tolerance Test (MMTT) is the most indicated.It attempts to assess the response produced by a typical meal. Natural foods have been used in a non-standardised manner24. Still, it is more advisable to use liquid formulas of known composition (approximately 40−50 g of complex carbohydrates, with proteins and fats, in a volume of 250−300 ml and an energy contribution of 250−400 kcal). Up to 50–80 % of individuals with neuroglycopenia present blood glucose levels below 50 mg/dl on the MMTT, this percentage being lower in asymptomatic patients (15%)9,27,28. There is no agreement on the test duration, hypoglycaemia cut-off points or interpretation in asymptomatic subjects. Despite these limitations, it is the test of choice because it is the most physiological and can help confirm the diagnosis of PHH, considering a blood glucose cut-off point of less than 60 mg/dl or 3.3 mmol/l.
The fasting test would be indicated exclusively in patients with hypoglycaemia a few months after surgery (less than six months) and/or during fasting to rule out insulinoma.
Imaging testsConsidering that PHH is a functional alteration, imaging tests are not indicated to locate the focus of insulin hypersecretion6,29,30. In cases of fasting hypoglycaemia, imaging tests such as CT, pancreatic MRI or ultrasound endoscopy could be considered, although the results are generally negative10,31–33. Pancreatic tumours are exceptional34.
TreatmentAcute treatment of hypoglycaemiaIf the patient is conscious, they should consume 15 g of glucose or fast-absorbing carbohydrates, which can be repeated after 15 min if there is no improvement. Subsequently, it is recommended to eat slower-absorbing carbohydrates. In case of treatment with acarbose, pure glucose should be administered.
Severe hypoglycaemia with loss of consciousness should be treated with glucagon (intranasal or intramuscular). Intranasal glucagon offers a more convenient route of administration and management, so it would be more recommended in these patients. If there is no improvement, the patient should be transferred to a hospital to administer intravenous glucose.
Dietary treatmentDiet modification is the mainstay of treatment and is beneficial for most patients35–37. It includes reducing carbohydrates, especially high-glycaemic carbohydrates, and dietary changes to delay gastric emptying (Table 2).
Dietary recommendations in postprandial hyperinsulinaemic hypoglycaemia.
| Eat in a relaxed environment, slowly, chewing all foods well. Rest lying down 15−30 min after main meals to increase the gastric emptying time. |
| The diet should be divided into 5−6 meals throughout the day, with these being small in volume. |
| Do not drink water or other liquids during meals but at least 30 min before and after each meal. Hyperosmolar liquids (soups, purées) at extreme temperatures (very cold or hot) encourage faster gastric emptying. |
| Only main meals should contain carbohydrates, while light meals and snacks will preferably provide proteins and fats. |
| Choose complex carbohydrates and whole grains: legumes, hummus, nuts, quinoa, oatmeal, whole wheat bread and pasta (cooked al dente), leafy vegetables, tomatoes, carrots, courgettes, aubergines, etc., and some fruits (apples, pears, green banana, citrus fruits, peach, kiwi, pomegranate, fruits of the forest). |
| Eliminate all sugars and carbohydrates with a high glycaemic load, such as sugar, fructose, polyols, soft drinks and juices, sweets, ice cream, biscuits and cookies, pastries, popcorn, sugary cereals, honey, jam, white bread, potatoes, sweet potatoes, pasta, rice, couscous, corn, tapioca, some fruits (dried fruits, plums, persimmons, cherimoyas, grapes, ripe banana, mango, pineapple). |
| Control carbohydrate portions: 15−30 g/meal, 5−15 g/snack. |
| Ensure adequate protein intake at each meal (60−80 g/day), adding protein modules (whey protein isolate) if necessary. |
| Include only healthy fats in each meal or snack: about 15 g/meal, 5 g/snack (do not exceed 3−4 g/100 kcal): oils, olives, avocado, nuts, seeds, dairy products, fatty fish (not fried). |
| In severe cases, raw corn flour (Maizena®) can be used at a dose of 5−10 g for each intake, diluted in water or milk at room temperature. Other interesting options, such as modified starches (Glycosade®) and maltodextrins, would be pending evaluation in the PHH. |
| Avoid caffeine and alcohol consumption and assess lactose tolerance. |
CH: carbohydrates; PHH: postprandial hyperinsulinaemic hypoglycaemia.
In a randomised study in patients with PHH, a carbohydrate-restricted (30% of total energy) and high-protein (30% of total energy) diet showed a significant reduction in peak postprandial glucose, an increase in glucose nadir, a reduction in insulin secretion, GLP-1 and GIP, as well as an increase in glucagon secretion compared to the conventional diet (55% of total energy in the form of carbohydrates and 15% of proteins)38.
Some dietary supplements (guar gum, pectin and glucomannan) increase the viscosity of foods, slow gastric emptying and delay glucose absorption, although tolerance is limited. These supplements have demonstrated efficacy in improving hypoglycaemia symptoms, although most studies have been carried out after gastro-oesophageal surgery, but not after BS.
Pharmacological treatmentPharmacological treatments are considered when symptoms cannot be managed with dietary modifications. The level of evidence of their efficacy is low, and none of them has a data sheet stating that it has been approved for use in PHH. The main mechanisms of action would be:
- -
Reduction of the postprandial rise in glucose (acarbose, SGLT1/SGLT2 inhibitors).
- -
Reduction of insulin secretion (calcium antagonists, diazoxide, somatostatin analogues, IL-1β receptor antagonist).
- -
Slowing of gastric emptying (GLP-1 analogues).
- -
Increased blood glucose (glucagon).
- -
Blockade of GLP-1 action (GLP-1 antagonists).
Acarbose: is an α-glucosidase hydrolase inhibitor that slows and reduces glucose absorption by preventing the breakdown of carbohydrates to monosaccharides, reducing postprandial excursions of glucose, insulin and GLP-139,40. The usual doses are 100−300 mg/day, starting at lower doses (25 mg before meals) due to its adverse gastrointestinal effects. In a multicentre study conducted by our group3, acarbose was the first therapeutic step. It achieved a partial response (50% reduction in the number of hypoglycaemia events and their severity) in 18% of patients.
Calcium antagonists: inhibit calcium-mediated insulin secretion. There is experience with verapamil (80 mg/12 h) and nifedipine (30 mg/d), with a partial response in 50% of patients3,41–43. The main adverse effect is hypotension.
Diazoxide: β-cell ATP-dependent potassium channel blocker. Doses of 50 mg/12 h up to 150 mg/8 h have been shown to achieve a partial response in 50% of patients3,44,45. Its use is limited by its adverse effects: oedema, nausea, hypotension and headache.
Somatostatin analogues: reduce gastric emptying, slow intestinal transit, and inhibit the secretion of gastrointestinal hormones, including GLP-1, insulin and postprandial vasodilation. We have short-acting analogues (octreotide) and long-acting (octreotide–Lar, lanreotide, pasireotide). In the GOSEEN multicentre study3, octreotide was the most effective drug, achieving partial response to hypoglycaemia in 38.4% of patients and clinical remission in 23%. In cases described in the literature, this remission has been maintained in the long term46. Pasireotide also prevents hypoglycaemia after MMTT but is associated with postprandial hyperglycaemia, with a 75 μg dose being the most appropriate47. These drugs have a high cost and side effects (diarrhoea, steatorrhoea, cholelithiasis, pain at the injection site and QT prolongation).
GLP-1 receptor antagonists: the GLP-1 receptor antagonist, exendin 9–39, is a drug that is only available in the investigational setting8,48. The PREVENT study showed an increase in glucose nadir and a reduction in insulin peak in 20% of patients with PHH after MMTT. The 60 mg dose in real life reduced hypoglycaemic events by up to 60% without the appearance of hyperglycaemia49.
GLP-1 receptor analogues: experience is very limited; isolated cases have been treated with liraglutide or exenatide50, with improvement in hypoglycaemia and reduction in glycaemic variability.
DPP-IV inhibitors: a study with sitagliptin did not show effectiveness in treating hyperglycaemia51.
XOMA358 is an investigational intravenous IG2 monoclonal antibody that induces insulin resistance by binding to the insulin receptor and inhibiting its autophosphorylation and signalling. A phase 2 study showed an increase in postprandial insulin peak, glucose nadir and fasting glucose of 20%52.
Other treatments tested in very short series or isolation include SGLT2 inhibitors (empagliflozin) together with an IL-1 receptor antagonist (anakinra)53, canaglifozin 300 mg/day (SGLT1/SGLT2 inhibitor)54 or the use of glucagon in a continuous subcutaneous infusion pump connected to a glucose sensor55.
One study has compared the effectiveness of different drugs, used sequentially, in 11 patients with PHH: acarbose 50 mg/8 h, sitagliptin 100 mg/day, verapamil 120 mg/day, liraglutide 0.6–1.2 mg/day and pasireotide 300 μg single dose. Only the treatment with acarbose and pasireotide effectively reduced postprandial hypoglycaemia during MMTT, although pasireotide produced sustained hyperglycaemia51.
Given the current data, we believe that the first step in the medical treatment of postprandial hypoglycaemia would be acarbose (Fig. 2). If there is no improvement and the patient is hypertensive, a calcium antagonist could be introduced. The second step would be the use of long-acting somatostatin analogues (octreotide-Lar, lanreotide), leaving pasireotide or diazoxide for refractory cases.
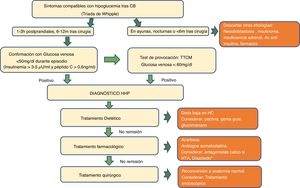
Diagnostic algorithm for hypoglycaemia after bariatric surgery. Ab: antibodies; BS: bariatric surgery; h: hours; CH: carbohydrates; m: months; PHH: postprandial hyperinsulinaemic hypoglycaemia; AHT: arterial hypertension; MMTT: mixed meal tolerance test.
*Although with less evidence, GLP-1 receptor agonists, SGLT2/SGLT1 inhibitor can be assessed. GLP-1 receptor antagonists and XOMA358 are still only available in the investigational setting.
Surgical treatment of hypoglycaemia after BS should be considered only when other dietary and pharmacological treatments have failed.
Since PHH is not due to pancreatic β-cell growth or hyperfunction but rather to changes in digestive anatomy and physiology induced by BS, partial or total pancreatectomy is not considered a therapeutic option. This technique carries high postoperative morbidity and mortality and a high level of recurrence of symptoms.
Surgical techniques will be aimed at delaying the rapid transit of the gastric reservoir to the intestine or at reconstructing the gastrointestinal tract to normal anatomy. In the first case, placement of a silicone ring, band56 and/or reduction of the diameter of the anastomosis and dilation of the gastric reservoir (candy-cane)3, with variable results. More recently, endoscopic techniques have been used to reduce the diameter of the gastrojejunal anastomosis, such as the transoral outlet reduction endoscopy (TORe) method, combined or not with argon plasma coagulation or this latter technique alone57,58.
Gastrostomy tube feeding in the remaining stomach normalises glucose levels, GLP-1, insulin and the incretin response6. For this reason, most authors support reversal surgery of the GBP to normal anatomy or to VSG to prevent weight regain. This type of surgery is effective in 80–88 % of patients59–62. This surgery is not exempt from complications: persistence of hypoglycaemia episodes, nausea and vomiting or limitations in oral tolerance, weight regain and gastro-oesophageal reflux. For this reason, it should be considered only if dietary and pharmacological treatment fails.
ConclusionsPostprandial hypoglycaemia is a frequent complication after BS and most episodes respond to dietary treatment. However, these hypoglycaemic episodes can be severe and give rise to symptoms of neuroglycopenia, which requires a complex approach ranging sequentially from dietary to pharmacological and even surgical measures. The recommendations presented are based on the limited current evidence on the subject and, above all, on the clinical experience of GOSEEN as a group of experts.
Conflicts of interestThe authors declare that they have no conflicts of interest.