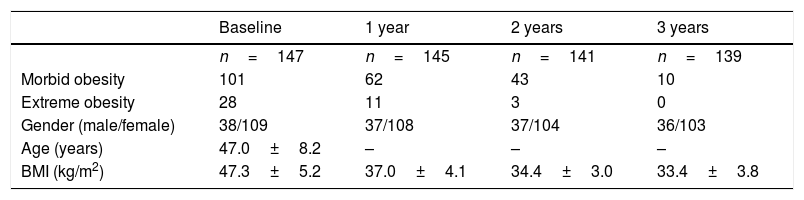The role of genetic variants of the CB2R gene in weight loss after a dietary intervention has been investigated in few studies, none of which has been conducted after bariatric surgery.
ObjectivesThe aim of this study was to assess the effect of the genetic variant (rs3123554) of the CB2R gene on cardiovascular risk factors and weight loss secondary to a biliopancreatic diversion.
DesignThe study simple consisted of 147 patients with morbid obesity. Biochemical and anthropometric parameters were measured at baseline and at each visit during 3 years (1, 2, and 3 years).
ResultsPercent excess weight loss, body mass index, weight, waist circumference, fat mass, blood pressure, fasting glucose, LDL cholesterol, total cholesterol, insulin, HOMA-IR, and triglyceride levels improved in both genotype groups. Decreases in fasting insulin levels and HOMA-IR were higher in non-A allele carriers as compared to A allele carriers.
ConclusionsOur data suggest that patients with morbid obesity who undergo bariatric surgery and carry the A allele of variant rs3123554 of the CB2R gene have greater weight. This allele has no influence on weight loss after surgery, but results in a lower decrease in insulin levels and insulin resistance.
El papel de las variantes genéticas del gen CB2R en la pérdida de peso después de una intervención dietética se ha investigado en pocos estudios, y hasta la fecha en ninguno tras cirugía bariátrica.
ObjetivosEl objetivo del presente estudio fue evaluar el efecto de la variante genética (rs3123554) del gen CB2R sobre los factores de riesgo cardiovascular y la pérdida de peso secundaria a una derivación biliopancreática.
DiseñoSe evaluó una muestra de 147 pacientes con obesidad mórbida. Los parámetros bioquímicos y antropométricos se estudiaron en la visita basal y en cada visita durante 3 años (1, 2 y 3 años).
ResultadosEl porcentaje de pérdida de peso en exceso, índice de masa corporal, peso, circunferencia de cintura, masa grasa, presión sanguínea, glucosa en ayunas, colesterol LDL, colesterol total, insulina, HOMA-IR y niveles de triglicéridos mejoraron en ambos genotipos tras la cirugía bariátrica. La disminución de los niveles de insulina en ayunas y HOMA-IR fue mayor en los portadores de alelos no A que en los portadores de alelos A.
ConclusionesNuestros datos sugieren que los pacientes con obesidad mórbida sometidos a una derivación biliopancreática y que son portadores del alelo A de la variante rs3123554 del gen CB2R presentan más peso. Este alelo no influye en la pérdida de peso tras la cirugía, pero sí en una menor disminución de los niveles de insulina y resistencia a la insulina.
The prevalence of obesity and its comorbidities is increasing, and constitutes a significant public health problem.1 This increase is attributable to environmental and genetic factors.2 The environmental factors are related to excessive calorie intake and diminished physical activity. Regarding the genetic factors, single nucleotide polymorphisms (SNPs) have been extensively associated with obesity. Some examples in the literature of obesity-related SNPs include those related to cannabinoid receptors, fat-related gene in obese patients, brain-derived neurotrophic factor, melanocortin subtype 4 receptor,3 etc.
In this context of influence exerted by both environmental and genetic factors, the role of the endocannabinoid system and its receptors is being studied as an important pathway related to obesity and patient response to the treatments used. The cannabinoid receptors are grouped into two families: CB1R and CB2R. These receptors belong to the G protein-coupled family of receptors and bind exogenous ligands derived from endogenous Cannabis sativa and endocannabinoids. The CB1R receptors are mainly located centrally in brain tissue, and their role in eating behavior has been well established.4 By contrast, the CB2R receptors are mainly expressed in peripheral tissues, including the cells of the immune system, and regulate the inflammatory response in various settings.5 Single nucleotide polymorphisms in this metabolic pathway have been frequently reported in the literature, and SNP rs1049353 of the CB1R gene, which results in G to A substitution in nucleotide position 1359, has been related to metabolic alterations in the Caucasian population.6 An SNP of the CB2R gene (rs3123554) has also been described. In this regard, Ketterer et al.7 have shown that carriers of the minor allele (A) of this SNP exhibit lower body weight together with lesser weight loss during dietary interventions. Apart from the cohort study of Ketterer et al.,7 two other nutritional intervention studies have explored the effect of this variant of the CB2R gene in relation to weight loss following hypocaloric diets.8,9 In the first study,8 obese patients who were not carriers of the A allele had a greater decrease in body weight, fat mass, insulin resistance and lipid parameters than those carrying the A allele, after an intervention consisting of mild calorie restriction for three months based on a Mediterranean diet. The more recent study9 examined the effect of two different low-calorie diets (moderate carbohydrate restriction versus normal carbohydrate intake). Carriers of the A allele lost less weight during the dietary intervention with the two low-calorie diets, while improvements in inflammatory parameters and glucose metabolism were less pronounced. To date, no studies have examined the effect of this genetic variant of the CB2R gene upon weight loss and the metabolic changes secondary to bariatric surgery in patients with morbid obesity.
The objective of the present study was to assess the effect of the genetic variant (rs3123554) of the CB2R gene upon cardiovascular risk factors and weight loss secondary to biliopancreatic diversion surgery (BPD).
Material and methodsStudy subjects and surgical procedureThe present study involved a total of 147 Caucasian obese patients without diabetes mellitus (DM) (109 women and 38 men), presenting a body mass index (BMI)≥35kg/m2 with associated comorbidities or a BMI≥40kg/m2. Exclusion criteria included severe liver or kidney disease, diabetes mellitus, malignant tumors, coagulopathy, gastrointestinal tract disease, and drug abuse. The following inclusion criteria were applied: a BMI≥40kg/m2 or a BMI≥35kg/m2 with associated comorbidities except diabetes mellitus and a history of failed weight loss with low-calorie diets before surgery. The patients underwent BPD with a non-randomised prospective observational study design (Table 1). The surgical technique consisted of a food loop of 175cm and a common final loop of 70cm, with partial gastrectomy and closing of the duodenal stump. The small intestine was sectioned at midlevel between the angle of Treitz and the ileocecal valve, followed by a Roux-en-Y gastroenterostomy in the distal intestinal loop and an end-to-side enteroileostomy of the proximal intestinal loop in the ileum 50–75cm before the ileocecal valve.
Anthropometric and biochemical parameters of the patients included in the study.
| Baseline | 1 year | 2 years | 3 years | |
|---|---|---|---|---|
| n=147 | n=145 | n=141 | n=139 | |
| Morbid obesity | 101 | 62 | 43 | 10 |
| Extreme obesity | 28 | 11 | 3 | 0 |
| Gender (male/female) | 38/109 | 37/108 | 37/104 | 36/103 |
| Age (years) | 47.0±8.2 | – | – | – |
| BMI (kg/m2) | 47.3±5.2 | 37.0±4.1 | 34.4±3.0 | 33.4±3.8 |
Of the 147 initial patients, 8 were non-morbidly obese with comorbidities. Morbid obesity: BMI40kg/m2 and <50kg/m2; extreme obesity>50kg/m2.
All procedures in this study were performed in accordance with the Declaration of Helsinki, and were approved by the local Ethics Committee (HCUVA-Committee-3/2016). Written informed consent was obtained from all the patients.
Clinical and biochemical parametersIn this study, all variables were recorded before surgery and after surgery at 1, 2 and 3 years. The following parameters were recorded: age, weight, height, the BMI, waist circumference (WC), fat mass through bioimpedance testing, percentage excess weight loss (%EWL), systolic and diastolic blood pressure, serum lipid levels (total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides), fasting glucose, insulin, insulin resistance based on the homeostasis model for insulin resistance (HOMA-IR), and associated morbidities (percentage of patients with hypertension and hyperlipidemia). The rs3123554 genotype of the CB2R gene was evaluated.
Body weight was measured with a precision of 25g using a calibrated scale (Omron, Los Angeles, CA, USA). The BMI was calculated as body weight (in kg) divided by height (in meters) squared. WC was measured using a flexible, non-elastic measuring tape (SECA, Birmingham, UK), while %EWL was calculated as: %EWL=preoperative weight−current weight×100/preoperative weight−ideal weight. Ideal weight was calculated based on an ideal BMI of 22kg/m2. Electrical bioimpedance was used to determine the fat mass with a precision of 50g (Akern, EFG, Milan, Italy).10 Blood pressure was measured twice after a 10-minute rest using a sphygmomanometer (Omrom, Los Angeles, CA, USA).
Blood samples were collected after an overnight fasting period of 12h. After centrifugation, the serum was stored at −80°C. Serum concentrations of total cholesterol, HDL-cholesterol and triglycerides were determined using an enzymatic colorimetric assay (Roche Diagnostics, Mannheim, Germany). The LDL-cholesterol levels were calculated using the Friedewald formula.11 Plasma glucose levels were measured using an automated glucose oxidase method (Glucose analyzer 2, Beckman Instruments, Fullerton, CA, USA). Insulin was measured by enzymatic colorimetry (Insulin, WAKO Pure Chemical Industries, Osaka, Japan),12 and insulin resistance was calculated based on HOMA-IR.13
Arterial hypertension and hyperlipidemia were diagnosed according to the standards of the National Cholesterol Education Program.14 Arterial hypertension and hyperlipidemia were also diagnosed in patients receiving hypotensive and lipid-lowering drugs at the time of surgery, respectively. The remission of hypertension or hyperlipidemia was considered when the baseline values corresponding to blood pressure or LDL-cholesterol were normal according to the standards,14 and medication was discontinued.
GenotypingGenomic DNA was extracted from 5ml of peripheral blood at the start of the study. The DNA was extracted from the leukocyte fraction using a commercial kit (Biorad, Los Angeles, CA, USA). The quantity and quality of DNA were measured using a NanoDrop ND-1000 spectrometer (Bio-Rad®, San Diego, CA, USA). The primers were designed with Sequenom Assay Design v4 (SEQUENOM, Inc., San Diego, CA, USA). Genotyping for polymorphism rs3123554 was carried out by real-time polymerase chain reaction (RT-PCR) analysis. This polymerase chain reaction was performed with 30ng of genomic DNA and 0.1–0.15μl of each of the primer oligonucleotides for rs670 (direct primer: 5′-ACGTTGGATGATTGTACCGAGGAGGGAACT-3′ and reverse: 5′-ACGTTGGATGGAGACACGTATTCTAGTCCC-3′ in an end volume of 2.5μl [Life Technologies thermocycler, Los Angeles, CA, USA]). The DNA was denatured at 85°C for 5min, followed by 45 cycles at 65°C for 15s, and the annealing at 58.1°C for 45s. The Weinberg equilibrium was calculated using a statistical test (chi-squared test), and the gene variant CB2R was found to be in Hardy–Weinberg equilibrium (p=0.36). The SPSS version 15.0 statistical package (SPSS, Chicago, IL, USA) was used throughout.
Statistical analysisSample size estimation was based on the effects upon weight loss using the frequency of the polymorphism (30%) in subjects with morbid and extreme obesity (n=130). All analyses were performed under a dominant genetic model with the rs3123554 A allele as the risk allele (AA+AG versus GG). The Kolmogorov–Smirnov test was used to determine variable distribution. Results were reported as the mean±standard deviation (SD). Other variables were analyzed using analysis of variance (ANOVA) in the case of variables with a normal distribution, or the Kruskal–Wallis test in the absence of normal data distribution. A two-way repeated measures ANOVA was used to assess the genotype effects. Multiple tests correction (Bonferroni correction) was used. Qualitative variables were analyzed using the chi-squared test, with Yates correction and the Fisher exact test. The chi-squared test was used to assess the Hardy–Weinberg equilibrium. Statistical significance was considered for p<0.05.
ResultsThe genotypic frequencies for polymorphism rs3123554 in the 147 patients (aged 47.0±8.2 years, 74.1% women) showed the following distribution: 34.0% (n=50) GG, 34.7% (n=51) GA and 31.3% (n=46) AA. The allelic frequency was G (0.51) and A (0.49). The gender distribution was similar in the different genotypes (GG, 22.0% [n=11] males and 78.0% [n=39] females; GA, 29.4% [n=15] males and 70.6% [n=36] females; and AA, 28.3% [n=13] males and 61.7% [n=23] females). The mean age was also similar in the different genotype groups (GG 47.1±5.1 years versus GA+AA 46.8±3.9 years; p=ns). Table 1 shows the characteristics of the patients included in the study.
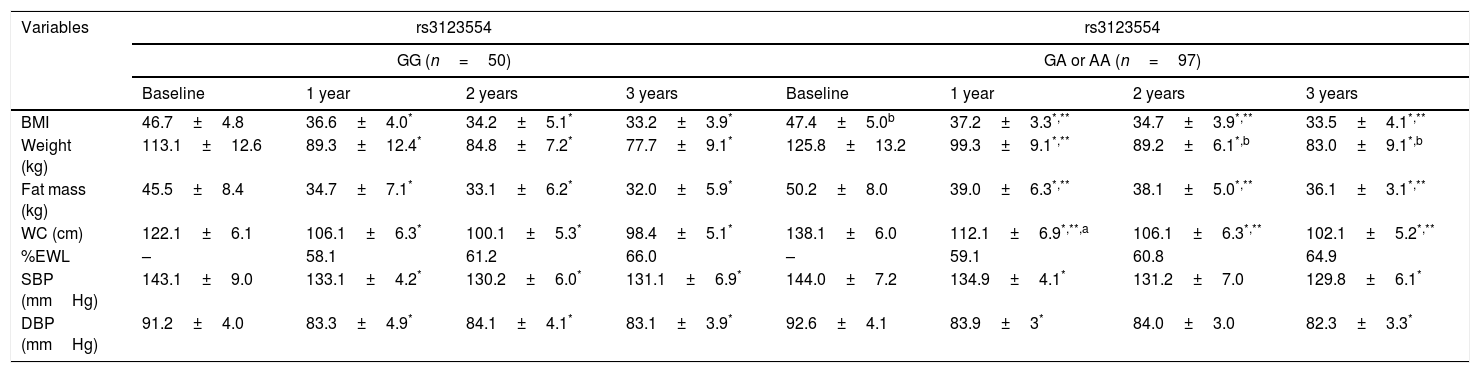
Table 2 shows the parameters of the patients before and after BPD. All the parameters showed a statistically significant decrease following surgery at 1, 2 and 3 years of follow-up. Preoperative differences were observed in weight, the BMI, fat mass and WC between genotypes, with higher values in the patients carrying the A allele. The improvements in these variables were similar in both genotypes, and all parameters were significantly different from the corresponding baseline values over the course of the three years. In both genotypes, %EWL showed significant improvement at all timepoints during follow-up.
Changes in anthropometric parameters rs3123554 (mean±standard deviation).
| Variables | rs3123554 | rs3123554 | ||||||
|---|---|---|---|---|---|---|---|---|
| GG (n=50) | GA or AA (n=97) | |||||||
| Baseline | 1 year | 2 years | 3 years | Baseline | 1 year | 2 years | 3 years | |
| BMI | 46.7±4.8 | 36.6±4.0* | 34.2±5.1* | 33.2±3.9* | 47.4±5.0b | 37.2±3.3*,** | 34.7±3.9*,** | 33.5±4.1*,** |
| Weight (kg) | 113.1±12.6 | 89.3±12.4* | 84.8±7.2* | 77.7±9.1* | 125.8±13.2 | 99.3±9.1*,** | 89.2±6.1*,b | 83.0±9.1*,b |
| Fat mass (kg) | 45.5±8.4 | 34.7±7.1* | 33.1±6.2* | 32.0±5.9* | 50.2±8.0 | 39.0±6.3*,** | 38.1±5.0*,** | 36.1±3.1*,** |
| WC (cm) | 122.1±6.1 | 106.1±6.3* | 100.1±5.3* | 98.4±5.1* | 138.1±6.0 | 112.1±6.9*,**,a | 106.1±6.3*,** | 102.1±5.2*,** |
| %EWL | – | 58.1 | 61.2 | 66.0 | – | 59.1 | 60.8 | 64.9 |
| SBP (mmHg) | 143.1±9.0 | 133.1±4.2* | 130.2±6.0* | 131.1±6.9* | 144.0±7.2 | 134.9±4.1* | 131.2±7.0 | 129.8±6.1* |
| DBP (mmHg) | 91.2±4.0 | 83.3±4.9* | 84.1±4.1* | 83.1±3.9* | 92.6±4.1 | 83.9±3* | 84.0±3.0 | 82.3±3.3* |
WC: waist circumference; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; %EWL: percentage excess weight loss.
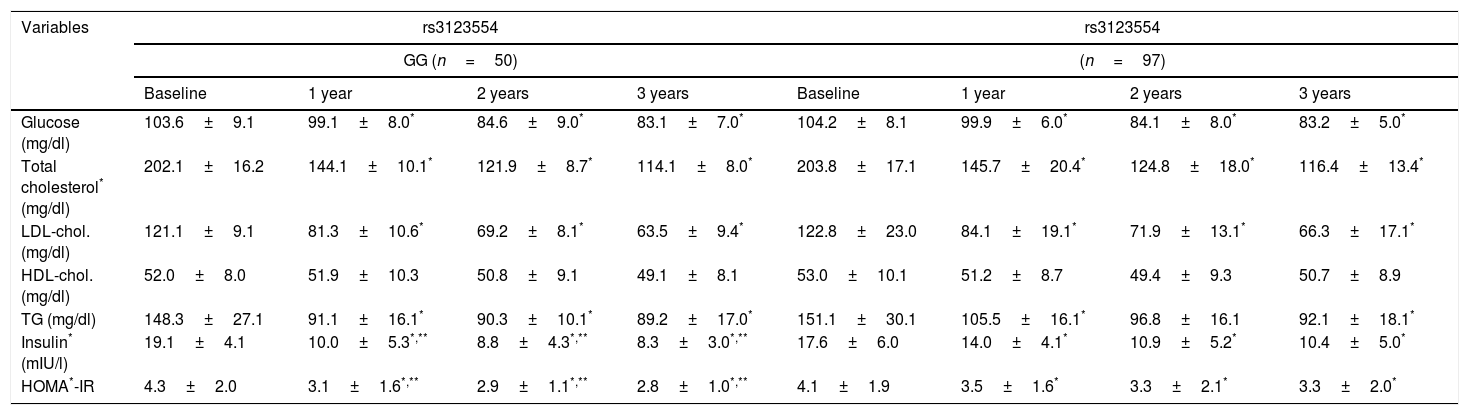
Table 3 shows the changes in the biochemical parameters. No significant preoperative differences were observed in serum glucose, HOMA-IR, insulin, total cholesterol, LDL-cholesterol, HDL-cholesterol or triglycerides between the two genotypes. Fasting glucose, total cholesterol, LDL-cholesterol and triglycerides decreased in both genotype groups during follow-up. However, the decrease in insulin and HOMA-IR, while significant in both genotypes, proved significantly greater in non-carriers of the A allele. The decrease in fasting insulin after the first year (delta: −9.1±1.4mIU/l vs. −3.6±1.3mIU/l; p=0.02), the second year (delta: −10.3±1.3mIU/l vs. −6.9±1.2mIU/l; p=0.01) and the third year (delta: −11.8±3.2mIU/l vs. −7.2±2.3mIU/l; p=0.02) was higher in non-carriers of the A allele than in carriers of the latter. The improvement in HOMA-IR in the first year (delta: −1.2±0.4mIU/l vs. −0.6±0.8mIU/l; p=0.02), second year (delta: −1.4±0.4mIU/l vs. −0.8±0.2mIU/l; p=0.01) and third year (delta: −1.5±1.1mIU/l vs. −0.8±0.6mIU/l; p=0.01) was also greater in non-carriers of the A allele than in carriers of the latter.
Biochemical parameters (mean±standard deviation).
| Variables | rs3123554 | rs3123554 | ||||||
|---|---|---|---|---|---|---|---|---|
| GG (n=50) | (n=97) | |||||||
| Baseline | 1 year | 2 years | 3 years | Baseline | 1 year | 2 years | 3 years | |
| Glucose (mg/dl) | 103.6±9.1 | 99.1±8.0* | 84.6±9.0* | 83.1±7.0* | 104.2±8.1 | 99.9±6.0* | 84.1±8.0* | 83.2±5.0* |
| Total cholesterol* (mg/dl) | 202.1±16.2 | 144.1±10.1* | 121.9±8.7* | 114.1±8.0* | 203.8±17.1 | 145.7±20.4* | 124.8±18.0* | 116.4±13.4* |
| LDL-chol. (mg/dl) | 121.1±9.1 | 81.3±10.6* | 69.2±8.1* | 63.5±9.4* | 122.8±23.0 | 84.1±19.1* | 71.9±13.1* | 66.3±17.1* |
| HDL-chol. (mg/dl) | 52.0±8.0 | 51.9±10.3 | 50.8±9.1 | 49.1±8.1 | 53.0±10.1 | 51.2±8.7 | 49.4±9.3 | 50.7±8.9 |
| TG (mg/dl) | 148.3±27.1 | 91.1±16.1* | 90.3±10.1* | 89.2±17.0* | 151.1±30.1 | 105.5±16.1* | 96.8±16.1 | 92.1±18.1* |
| Insulin* (mIU/l) | 19.1±4.1 | 10.0±5.3*,** | 8.8±4.3*,** | 8.3±3.0*,** | 17.6±6.0 | 14.0±4.1* | 10.9±5.2* | 10.4±5.0* |
| HOMA*-IR | 4.3±2.0 | 3.1±1.6*,** | 2.9±1.1*,** | 2.8±1.0*,** | 4.1±1.9 | 3.5±1.6* | 3.3±2.1* | 3.3±2.0* |
Chol.: cholesterol; HOMA-IR: homeostasis model assessment; TG: triglycerides.
Lastly, in this group of morbidly obese patients without diabetes mellitus, remission among those diagnosed with arterial hypertension and hyperlipidemia before treatment proved similar in the two genotypes. Among the patients with the GG genotype, a total of 25 (50%) had dyslipidemia, while 47 with genotype GA+AA had high lipid levels (48.5%). Three years after surgery, only 4% and 3.1% had high lipid levels, respectively.
With regard to arterial hypertension, among the patients with the GG genotype, 14 (28.0%) had hypertension, versus 26 with genotype GA+AA (26.8%). Three years after surgery, only 2.0% and 3.1% had high blood pressure levels, respectively.
DiscussionThis study has established an association between the A allele of SNP rs3123554 within the CNR2 gene and an increased BMI and other adiposity parameters among patients with morbid obesity eligible for bariatric surgery. In addition, obese subjects not carrying the A allele showed a better response on the part of serum insulin concentration and insulin resistance (HOMA-IR) than carriers of the A allele after BPD at all follow-up timepoints.
Taking into account that obesity is a major health problem and a first-order cardiovascular risk factor, a primary objective of interventions in this group of patients is to secure sustained body weight loss over time. Genetic-environmental interactions are of great interest in developing more personalized care in daily medical practice, and in this regard SNPs have been identified that are associated with obesity risk and/or the modification of associated cardiovascular risk factors after dietary intervention15 or bariatric surgery.16 The above observations led us to investigate the effects of SNP rs3123554 within the CNR2 gene upon weight loss and metabolic changes three years after BPD. No studies to date have evaluated the interaction of this polymorphism with the effects of bariatric surgery of any kind. Surprisingly, the minor allele of rs3123554 (A) was associated with greater body weight and a lesser improvement of some of the metabolic parameters. In recent years, the endocannabinoid system has been associated with inflammatory pathways,17 and moreover there is growing evidence of the role of inflammation in the pathogenesis of cardiovascular risk among obese individuals.18,19 As mentioned above, CB2R has been known for some time as the peripheral cannabinoid receptor isoform. There is evidence of CB2R expression in different brain areas.20 The central presence of this receptor may explain the findings of our study, which relate the above-mentioned polymorphism to certain obesity parameters such as fat mass, the BMI and WC in carriers of the A allele. On the one hand, CB2R activation in humans is known to influence dietary behavior,21 and on the other CB2R expression could regulate endocannabinoid levels, thereby conditioning craving and dietary reward behavior through the corresponding neuronal circuit.22 These situations could influence the observed differences in weight.
The different metabolic responses between the two genotypes deserve special attention. Surprisingly, the A allele of the variant rs31235554 led to a lesser decrease in the parameters related to carbohydrate metabolism (insulin levels and HOMA-IR). Two hypotheses can be proposed to explain this. Firstly, a central hypothesis refers to the observations of Ketterer et al.,7 who found that carriers of the A allele have decreased sensitivity to brain insulin, and in this regard it should be noted that brain insulin sensitivity facilitates body weight loss during calorie restriction.19 On the other hand, these previous studies7 have demonstrated a reduced effect related to this genetic variant in the Theta band of the brain. Considering that insulin sensitivity in the brain determines the effectiveness of dietary interventions in terms of weight loss,23 it could be postulated that decreased brain insulin sensitivity in A allele carriers may be related to greater weight and to a lesser improvement in insulin levels and insulin resistance after surgery.
A second hypothesis for explaining these findings could be called the “peripheral theory”, because CB2R has been isolated in some target organs related to the control of glycemic metabolism such as the liver, adipose tissue and skeletal muscle.24 In obese individuals, all of these tissues are involved in insulin resistance and lipid metabolism. Furthermore, the peripheral effect of CB2R upon metabolism in different pathways has already been demonstrated. Two examples of these peripheral effects are the role of CB2R polymorphism rs35761398 in earlier age at menarche in patients carrying the Q63 allele25 and the role of SNPs rs3003336, rs2501431, rs2502992 and rs2501432 of the CB2R gene in the etiology of osteoporosis, suggesting that CB2R may play an important role in bone density and osteoporosis among postmenopausal women.26 Lastly, both hypotheses could be combined to explain our results, the difference in body weight found in the baseline data possibly being due to peripheral metabolic mechanisms, while after surgery, the altered brain activity inherent in the reward system and the different central sensitivity to insulin could explain the biochemical data.
Another interesting observation is that before surgery, body weight and the associated clinical variables were greater in carriers of the A allele, thus explaining why during follow-up, improvements in insulin sensitivity were less pronounced in these patients despite similar weight loss. Clinical practice possibly causes us to apply surgical techniques with a greater impact upon weight loss or insulin resistance in carriers of the A allele, and to monitor their carbohydrate metabolism more closely.
The limitations of our study include, for example, the lack of analysis of some uncontrolled factors that could have influenced our results (epigenetic and hormonal status, other unknown environmental factors, etc.). In addition, the lack of a control cohort without surgery could imply bias. Likewise, interactions with other genes were not investigated in our study; for example, rs3123554 is located only 1.5kb from the FUCA1 gene and is related to metabolic diseases.27
In conclusion, our data suggest that patients with obesity eligible for bariatric surgery carrying the A allele of variant rs3123554 of the CB2R gene exhibit greater body weight, and in the event of similar weight losses after bariatric surgery, the improvements in insulin levels and insulin resistance are less pronounced. These results should be assessed in future studies in order to analyze the effect of these laboratory differences upon more relevant clinical variables in the comorbidities of patients with morbid obesity.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: de Luis D, Garcia Calvo S, Primo D, Izaola O, Pacheco D. El polimorfismo del gen del receptor cannabinoide subtipo 2 (CB2R) rs3123554 está asociado con los cambios metabólicos tras la derivación biliopancreática. Endocrinol Diabetes Nutr. 2019;66:157–163.