The risk of recurrence in papillary thyroid carcinoma (PTC) is likely related to the amount of tumour in the metastatic lymph node (LN). Therefore, the current TNM classification (N0/N1) make it necessary to find a method to quantify the LN metastasis (LNM). We propose that the quantitative molecular assay One-Step Nucleic-Acid Amplification (OSNA), which measures the number of cytokeratin-19 (CK-19) mRNA copies as a marker of LNM, could play this role. Our objective was to describe the characteristics of the LNs from PTC, and to compare the morphological characteristics that have been claimed as criteria for metastatic burden with OSNA.
Patients and methodProspective study of LNs from 42 patients. All of the LNs were measured, weighed and analysed by OSNA and also by imprint cytology.
ResultsA total of 573 LNs were included, 187 (32.6%) of them were OSNA-positives. The global consistency between cytology and OSNA was 87.4%. Significant differences were observed in the CK-19 copy number between the LNMs<0.2cm and those >3cm, as well as between those from 0.2 to 3cm with respect to those >3cm, but not between those <0.2cm and those between 0.2 and 3cm. The total tumour load per neck dissection showed no differences based on whether there were ≤5 or >5 LNMs.
ConclusionsIn our series the LNMs >3cm show an increased tumour load, but it is unclear if it is necessary to sub-classify the smaller ones as well as the relevance of the number of metastatic nodes according to the cut-off of 5 nodes. We consider that the OSNA analysis avoids the bias of nodal histology and allows for a greater understanding of its real oncological potential.
La recurrencia en el carcinoma papilar de tiroides (CPT) parece relacionarse con la cantidad de tumor en el ganglio metastásico. Por tanto, la actual clasificación TNM (N0/N1) hace necesaria la búsqueda de un método capaz de cuantificar dicha metástasis. Proponemos que el análisis molecular cuantitativo One-Step Nucleic-Acid Amplification (OSNA), que mide el número de copias de citoqueratina-19 como marcador de metástasis ganglionar, puede jugar un papel en ello. Los objetivos de nuestro trabajo fueron describir las características de los ganglios linfáticos en CPT y comparar con OSNA los hallazgos morfológicos que han sido propuestos como criterios de carga metastásica ganglionar.
Pacientes y métodoEstudio prospectivo de los ganglios linfáticos de 42 pacientes. Todos ellos fueron pesados, medidos y analizados mediante OSNA, así como mediante impronta citológica.
ResultadosSe obtuvo un total de 573 ganglios, resultando positivos por OSNA 187 (32,6%). La concordancia global entre OSNA y citología alcanzó el 87,4%. Se observaron diferencias significativas en el número de copias de citoqueratina-19 entre los ganglios <0,2 y los >3cm, así como entre los de 0,2-3cm respecto a los >3cm, pero no entre los <0,2 y aquellos entre 0,2-3cm. La carga tumoral total por linfadenectomía no mostró diferencias según el punto de corte de 5 ganglios metastásicos.
ConclusionesEn nuestra serie las metástasis ganglionares >3cm muestran mayor carga tumoral, pero se pone en duda la necesidad de subclasificar aquellas de menor diámetro, así como el valor del número de ganglios metastásicos según el punto de corte de 5 ganglios. Consideramos que el análisis mediante OSNA suple las limitaciones de la histología convencional y aporta mayor información acerca de su potencial oncológico real.
The principal manner in which papillary thyroid carcinoma (PTC) spreads is through the lymphatic system. When diagnosed, 35–85% of cases have cervical lymph node metastases (LNM).1 However, it is becoming evident that the presence of LNM is only an indicator of a poor prognosis when there is an abundant amount of tumour tissue in the lymph node (LN).2 Therefore, the simple dichotomised classification according to the TNM staging system3 seems inefficient. In this system N0 refers to the absence of LNM while N1 refers to the presence of LNM, without taking into consideration the nodal tumour burden.
Numerous studies have attempted to objectively characterise this metastatic tumour load.4–8 In this sense, both the American Thyroid Association (ATA) and the College of American Pathologists (CAP) suggest adding information to the pathological report regarding the number of LNs analysed, the number of affected LNs, the size of the largest metastatic focus and the presence of extranodal extension (ENE).9–11 In turn, the TNM staging system recommends including both the size of the largest metastatic deposit as well as the total size of the affected LN.3 The fact that certain guidelines and recommendations mention “metastatic LN size” while others refer to the “size of the metastatic focus” leads to ambiguity and to a lack of consensus in how these concepts are used.
Another aspect that leads to confusion is the use of the terms “micrometastasis” and “macrometastasis”. ATA guidelines define the use of micrometastasis as a metastatic focus <0.2cm, based on an extrapolation from breast cancer,9 whereas the TNM staging classification recognises that there are currently no pertinent cut-off values applicable to these measurements in the case of PTC.3
Lastly, LNM in PTC shows histological peculiarities such as cystic degeneration or multifocality which further distort the real quantification of LN status.
For all of these reasons, it is necessary to find a standardised assessment method for the real quantification of the LNM tumour load.
In recent years, the One-Step Nucleic Acid Amplification (OSNA) molecular assay has been widely introduced in the analysis of LNM, especially for assessing sentinel lymph nodes (SLN) in breast cancer.12,13 OSNA is based on a real-time quantitative technique capable of detecting the cytokeratin 19 (CK-19) mRNA in the entire LN.14,15 The expression of the CK-19 protein is characteristic of PTC.16 Previous studies were conducted to validate the OSNA assay for the analysis of LN in PTC by our group in collaboration with other centres.17,18 This study allowed us to subsequently demonstrate the usefulness of OSNA in the intraoperative analysis of the SLN in the context of PTC for the first time.19 Consequently, we consider that the use of OSNA could solve the limitations of conventional LN analysis, providing an objective measurement of the metastatic tumour load. Accordingly, the objectives of this work are: (1) to describe the characteristics of the LNs in patients with PTC and (2) to compare the morphological characteristics that in recent years have been claimed as criteria for metastatic burden with the quantitative results provided by OSNA in a series of more than 550 LNs.
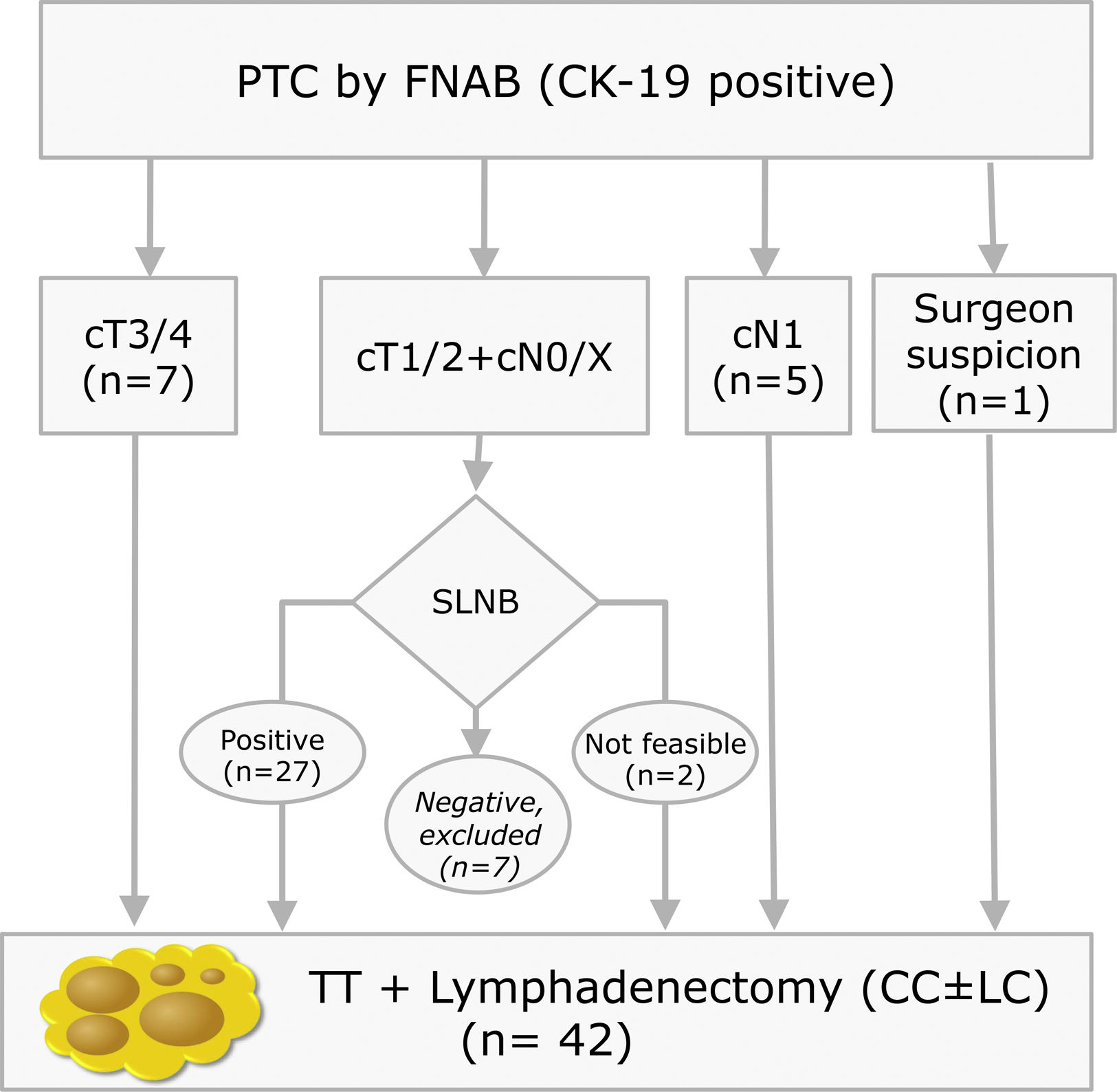
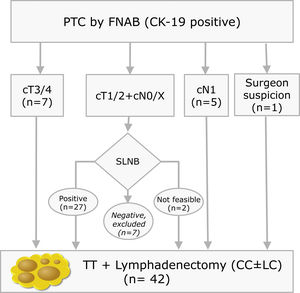
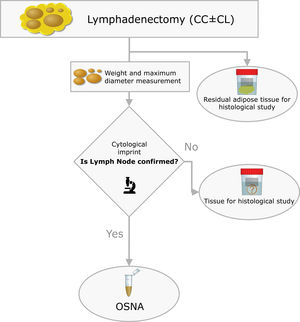
Materials and methodsThis is a prospective study with LNs obtained from lymphadenectomies of patients with PTC from 2013 to 2016 that met the criteria shown in Fig. 1. In our centre, selective sentinel lymph node biopsy (SLNB) is a practice that has been applied on patients with PTC at low/intermediate risk for the last 10 years. Positive results from the sentinel lymph node indicate the need to perform lymph node dissection, as such a part of the patients included meet this criterion. The rest of the inclusion criteria match those proposed by the ATA guidelines.9 All patients accepted and signed the informed consent form, which was approved by the ethics committee of our institution on 07/01/13 (reference number PR (SC) 192/2013).
Inclusion criteria distribution for the 42 patients enrolled in the study (PTC: Papillary Thyroid Carcinoma; FNAB: Fine Needle Aspiration Biopsy; CK-19: Cytoqueratin-19; SLNB: Sentinel Lymph Node Biopsy; TT: Total Thyroidectomy; CC: Central Compartment; LC: Lateral Compartment; cT and cN according to TNM Classification of Malignant Tumours, 7th Ed, reference 3).
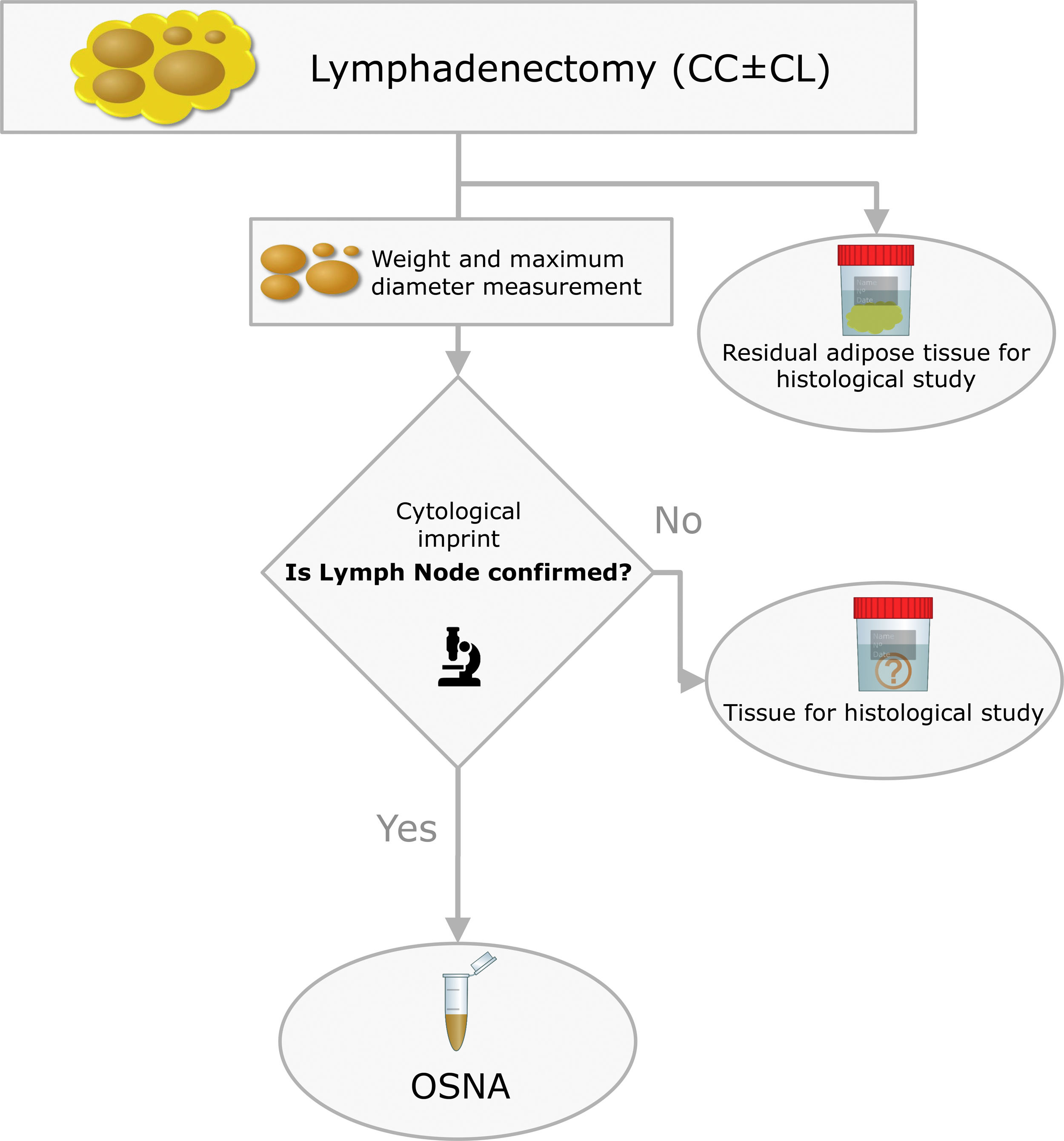
The surgical specimens were sent to the Pathology Laboratory where an immediate macroscopic study was performed to achieve the greatest extent of fresh LN, preserving the residual adipose tissue for conventional histology. All of the LNs identified were measured and weighed. A central section was made in each node, and two cytological imprints were taken. One was stained with Diff-Quik® (Química Clínica Aplicada, S.A., Spain) and the other with Papanicolaou (Merck KGaA, Germany). This cytology had a two-fold objective: (1) to have a morphological verification of the sample identified as LN, given that the entire tissue was analysed by OSNA and it was not possible to obtain a subsequent histological section, and (2) to evaluate the consistency between the molecular analysis and the morphology (Fig. 2).
The OSNA assay followed the methodology described in previous work (19). Briefly, the lymph node tissue was processed by mixing it with Lynorhag buffer to then be homogenised. From the resulting sample we pipetted 1mL, followed by centrifugation at 10,000g for 1min. The supernatant was diluted with Lynorhag buffer and analysed with the RD-100i-OSNA system (Sysmex Corp., Japan). OSNA reports the results in three categories: negative, which includes absolute negative (<100copies/μL) and isolated tumour cells – ITC – (100–250); micrometastasis – O_mM – (251–5000) and macrometastasis – O_MM – (>5000). Likewise, the validation of the OSNA assay in PTC that our group performed defined superimposable cut-offs.17,18
Statistical analysisThe data were summarised using mean (standard deviation) or median (interquartile range) for continuous variables and the proportion or frequency for categorical variables. A log transformation was used for non-normally distributed data before proceeding to the t-test. A chi-square test was used to compare frequencies. The parametric Student's t-test and ANOVA test or the nonparametric Mann–Whitney-U-test and the Kruskal–Wallis test were used to compare continuous variables. A Tukey test or a post hoc Conover–Inman test for multiple pairwise comparisons between groups was performed following a significant ANOVA or Kruskal–Wallis test, respectively. Correlations between diameter and weight and the nodal tumour load were analysed using the Pearson's correlation coefficient. P-values≤0.05 were considered statistically significant.
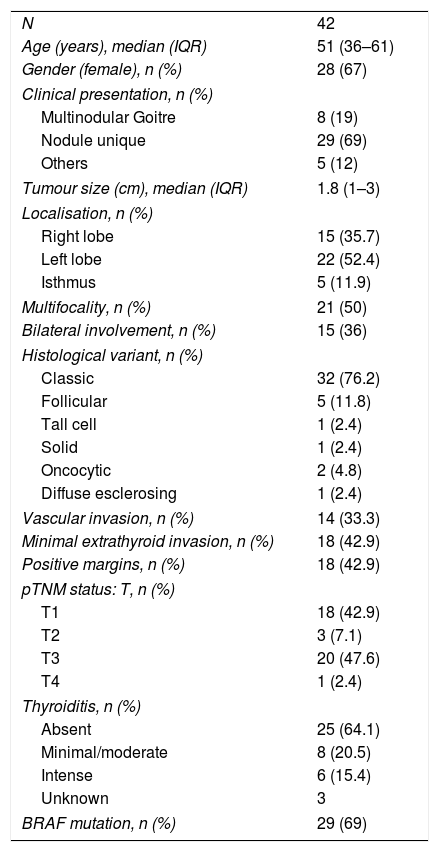
ResultsAnalysis of all LNsA total of 42 lymphadenectomies were included (Fig. 1), corresponding to 28 women and 14 men, with a mean age of 49±15 years old. The clinical–pathological characteristics are summarised in Table 1. After examining the specimens from the neck dissection, a total of 608 nodes were isolated, 35 of which (5.7%) were ruled out due to the fact that the lymphatic nature was not verified in the cytology and they were determined to be 21 adipose nodes, three thyroid nodes, 10 parathyroid glands and one thymic node. The remaining 573 nodes (94.2%) were compatible with LN. In the residual adipose tissue, another 409 millimetric LNs were identified, which initially went unnoticed due to their small diameters. Fourteen of the 409 (3.4%) were positives, with a mean diameter of 0.2cm (0.05–0.5cm). In no case would the positivity of these LNs have modified the patients’ staging. In seven patients, 20 additional LNs included in the thyroidectomy pieces were identified, with four of them positive. Therefore, considering the initially isolated fresh LNs, as well as those subsequently identified after the examination of the residual fat, and those included in the thyroidectomy, a total of 1002 LNs were obtained. This results in a definitive mean of 23.8LN/patient.
Clinic-pathological characteristics of included patients.
| N | 42 |
| Age (years), median (IQR) | 51 (36–61) |
| Gender (female), n (%) | 28 (67) |
| Clinical presentation, n (%) | |
| Multinodular Goitre | 8 (19) |
| Nodule unique | 29 (69) |
| Others | 5 (12) |
| Tumour size (cm), median (IQR) | 1.8 (1–3) |
| Localisation, n (%) | |
| Right lobe | 15 (35.7) |
| Left lobe | 22 (52.4) |
| Isthmus | 5 (11.9) |
| Multifocality, n (%) | 21 (50) |
| Bilateral involvement, n (%) | 15 (36) |
| Histological variant, n (%) | |
| Classic | 32 (76.2) |
| Follicular | 5 (11.8) |
| Tall cell | 1 (2.4) |
| Solid | 1 (2.4) |
| Oncocytic | 2 (4.8) |
| Diffuse esclerosing | 1 (2.4) |
| Vascular invasion, n (%) | 14 (33.3) |
| Minimal extrathyroid invasion, n (%) | 18 (42.9) |
| Positive margins, n (%) | 18 (42.9) |
| pTNM status: T, n (%) | |
| T1 | 18 (42.9) |
| T2 | 3 (7.1) |
| T3 | 20 (47.6) |
| T4 | 1 (2.4) |
| Thyroiditis, n (%) | |
| Absent | 25 (64.1) |
| Minimal/moderate | 8 (20.5) |
| Intense | 6 (15.4) |
| Unknown | 3 |
| BRAF mutation, n (%) | 29 (69) |
IQR: interquartile rang; BRAF: B-Raf proto-oncogene, serine/threonine kinase; pTNM according to TNM Classification of Malignant Tumours, 7th Ed, reference 3.
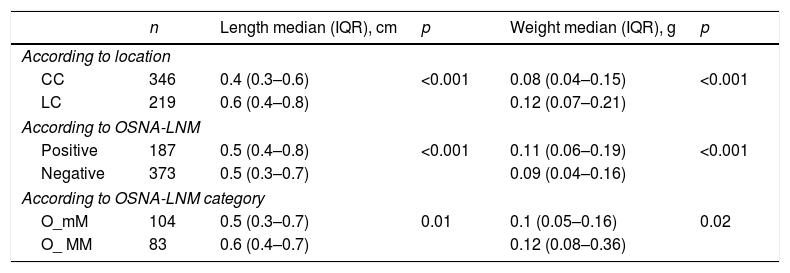
For the 573 LNs that could be analysed by OSNA, we obtained a mean of 13.6±8 LNs per patient. Of these, 346 (60.4%) corresponded to the central compartment (CC) and 219 (38.2%) to the lateral compartment (LC), while the location could not be established in eight cases. The median weight per LN was 0.09g (0.05–0.17) with a minimum of 0.01 and a maximum of 7.7g. The median diameter was 0.5cm (0.3–0.7) per LN, with a minimum of 0.04 and a maximum of 5cm. The LNs of the LC had a significantly larger size and weight than the LNs of the CC (Table 2).
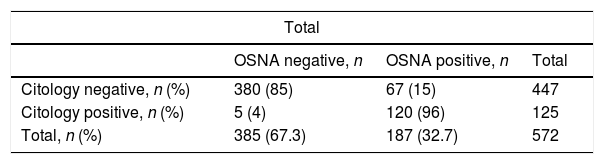
Analysis of the OSNA-positive LNsOne hundred twenty-five of the 573 (21.8%) LNs were positive in the cytological study, with no differences concerning the staining method (Diff-Quik® or Papanicolaou), and 187 (32.6%) were positive with the OSNA study (p<0.001). The cytology study and molecular analysis were consistent in 501 (87.4%) LNs. Table 3 includes the description of the discrepancy in the remaining 72 (12.6%) LNs. The biggest discrepancy between the two methods comes from the LNs with O_mM (p<0.001).
Discrepancies between cytological and molecular results.
| Total | |||
|---|---|---|---|
| OSNA negative, n | OSNA positive, n | Total | |
| Citology negative, n (%) | 380 (85) | 67 (15) | 447 |
| Citology positive, n (%) | 5 (4) | 120 (96) | 125 |
| Total, n (%) | 385 (67.3) | 187 (32.7) | 572 |
| When OSNA is absolute negative | |||
|---|---|---|---|
| OSNA− | OSNA+ | Total | |
| Citology− | 365 | 0 | 365 |
| Citology+ | 1 | 0 | 1 |
| Total | 366 | 0 | 366 |
| When OSNA shows isolated tumoral cells | |||
|---|---|---|---|
| OSNA− | OSNA+ | Total | |
| Citology− | 15 | 0 | 15 |
| Citology+ | 4 | 0 | 4 |
| Total | 19 | 0 | 19 |
| When OSNA shows micrometastases | |||
|---|---|---|---|
| OSNA− | OSNA+ | Total | |
| Citology− | 0 | 57 | 57 |
| Citology+ | 0 | 47 | 47 |
| Total | 0 | 104 | 104 |
| When OSNA shows macrometastases | |||
|---|---|---|---|
| OSNA− | OSNA+ | Total | |
| Citology− | 0 | 10 | 10 |
| Citology+ | 0 | 73 | 73 |
| Total | 0 | 83 | 83 |
OSNA: One-Step Nucleic-Acid Amplification.
Of the 187 OSNA-positive LNM, 71% corresponded to the CC and 29% to the LC. The OSNA-positive LNMs were of a significantly larger size and weight than the negative LNs (Table 2). Significant differences were also found in the length and the weight depending on whether they were classified as O_mM or O_MM (Table 2).
Length and weight of the lymph nodes analysed with OSNA.
| n | Length median (IQR), cm | p | Weight median (IQR), g | p | |
|---|---|---|---|---|---|
| According to location | |||||
| CC | 346 | 0.4 (0.3–0.6) | <0.001 | 0.08 (0.04–0.15) | <0.001 |
| LC | 219 | 0.6 (0.4–0.8) | 0.12 (0.07–0.21) | ||
| According to OSNA-LNM | |||||
| Positive | 187 | 0.5 (0.4–0.8) | <0.001 | 0.11 (0.06–0.19) | <0.001 |
| Negative | 373 | 0.5 (0.3–0.7) | 0.09 (0.04–0.16) | ||
| According to OSNA-LNM category | |||||
| O_mM | 104 | 0.5 (0.3–0.7) | 0.01 | 0.1 (0.05–0.16) | 0.02 |
| O_ MM | 83 | 0.6 (0.4–0.7) | 0.12 (0.08–0.36) | ||
OSNA: One-Step Nucleic-Acid Amplification; LNM: Lymph Node Metastasis; CC: Central Compartment; LC: Lateral Compartment; O_mM: micrometastasis by OSNA; O_MM: macrometastasis by OSNA; IQR: interquartile rang.
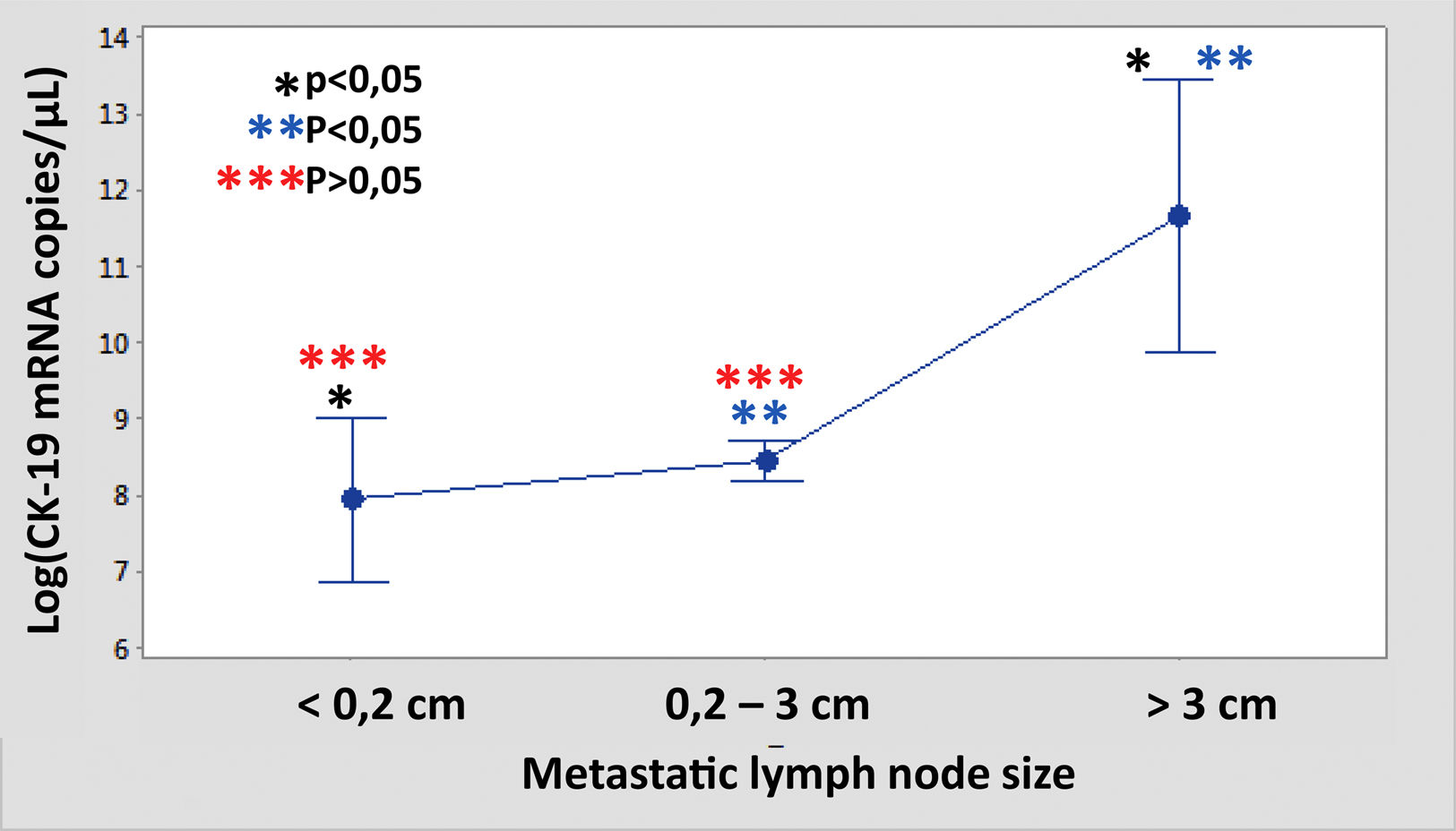
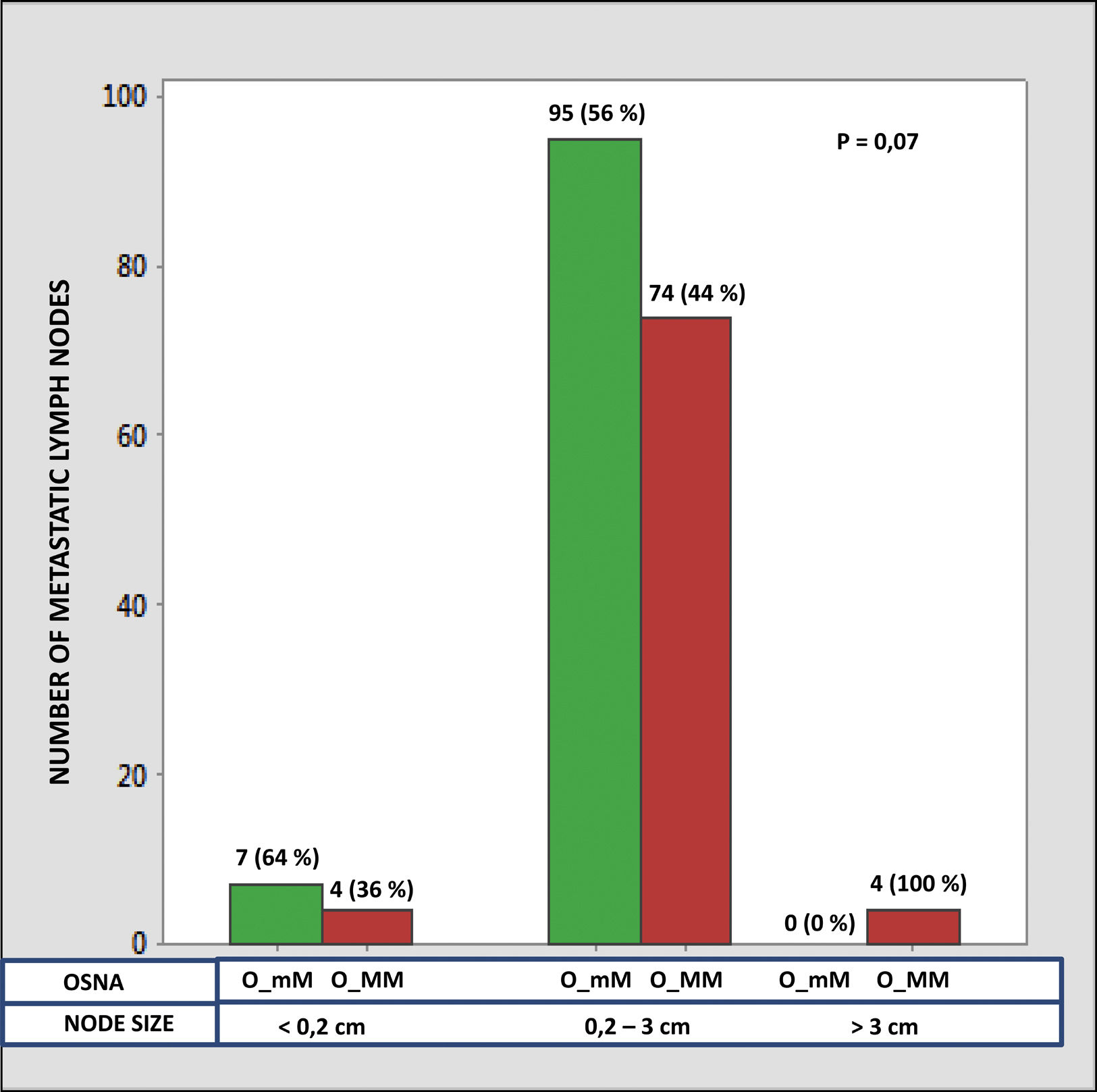
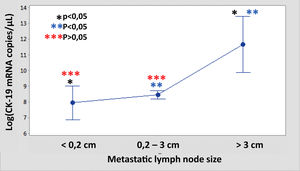
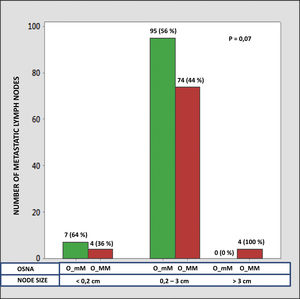
The median number of CK-19 mRNAcopies/μL per LNM was 4400 (1100–120,000) with a minimum of 250 and a maximum of 1,500,000. A significant correlation was detected between the weight (R=0.28; p<0.001) and the diameter (R=0.23; p<0.001) of the LNM with the tumour load, although the coefficient of determination (R2) was less than 0.1 in both cases. Significant differences were observed in the copy number between the LNM<0.2cm and those >3cm, as well as between those from 0.2 to 3cm with respect to those >3cm, but not between those <0.2cm and those between 0.2 and 3cm (Fig. 3). As Fig. 4 shows, 4 of the 11 (36%) LNMs of less than 0.2cm and 74 of the 169 (44%) of the group measuring between 0.2 and 3cm had O_MM. These differences were not significant. All the 4 LNMs >3cm corresponded to O_MM.
Lastly, the total tumour load (TTL) per neck dissection, defined as the sum of the CK-19 mRNA copies of all LNMs per patient, was analysed. The median TTL per case was 31,930 CK-19copies/μL with a minimum of 250 and a maximum of 2,101,760. There were no differences based on whether there were ≤5 or >5 LNMs (p=0.06). The relationship between the TTL and the neck dissection, based on whether there was an LNM>3cm or if all LNM were smaller, was at the limit of the significance (p=0.05).
Discussion/ConclusionThis work analysed more than 550 LNs of lymphadenectomies from patients with PTC. The first objective was to describe the morphological characteristics of the LNs. The results corroborate that the LNMs had a greater weight and diameter than the LNs without metastasis. The data additionally, though not so clearly, confirmed that both parameters are greater in the O_MM with respect to the O_mM. Likewise, we can see that the LNs from the LC are larger and have a greater weight than those from the CC.
The 2009 ATA guidelines classified the presence of LNM as an intermediate risk of recurrence.20 The updated version in 2015 clarifies some of the characteristics of these LNMs in order to classify the risk more precisely.9 In recent years it has become clear that the mere presence of LNM is not related to the prognosis of the disease and that the metastatic load is what indicates the risk of recurrence. With this aim, Randolph et al. defined four categories of LNM based on size. Using this classification, they established two risk groups according to size and number.2 The second objective of our work was to analyse if these parameters are accurately linked to the LN tumour load by means of the quantitative analysis provided by OSNA. In relation to the size of LNM, Randolph et al. defined micrometastasis (<0.2cm) or small metastasis (0.2–1cm) as low risk and LNMs>3cm as high risk, while the LNMs between 1 and 3cm were not included in either group.2 A 3-cm cut-off has been also suggested by other authors.21,22 We have been able to confirm that the LNMs>3cm have a significantly greater number of CK-19 copies than those with a smaller diameter. On the other hand, in the LNMs<3cm we did not find any differences in the tumour load with respect to the 0.2-cm cut-off. In the analysis with OSNA, the presence of O_MM is not significantly greater in LNMs<0.2cm with respect to those of 0.2–3cm (36% vs. 44%), while in 100% of the LNMs>3cm we observe O_MM. From these we can deduce that the LNM>3cm show an increased tumour load, but it is not clear if it is necessary to sub-classify those with a smaller size and if the cut-off at 0.2cm is a useful prognosis for defining micrometastasis in PTC. One of the reasons for confusion in establishing the cut-off between micrometastasis and macrometastasis is the ambiguity in the literature regarding if these points refer to the LN size or to the size of the metastatic focus inside the LN.23–25 The LNMs in the PTC have some particular features that make it so that the two concepts are not equivalent, as we have shown with the low correlation in this work. Specifically, there are two elements that we must take into consideration. First, there are frequently multiple tumour foci in the LN. Therefore, exclusively considering the largest could lead us to minimise the real amount of metastasis. Second, the cystic degeneration of the metastasis is common, given that there could be a large metastatic focus where the real extent of the tumour is minimal. In this case, assessing the size of the focus could lead us to maximise its real oncogenic potential. We propose that molecular analysis can have a role specifically in this case, providing precise and quantifiable information.
The number of LNMs has also been proposed as a criterion to define the high or low risk of recurrence. Randolph et al. propose a cut-off of 5 LNMs to differentiate between low and high risk.2 In our series, we have not been able to demonstrate differences in the TTL in relation to that cut-off, and, thus, the robustness of this criterion concerning the tumour load analysis. In this sense, some authors have proposed other cut-offs5,26–28 and other variables, such as the ratio between positive and total LNs.29–31
Although OSNA is a highly efficient quantitative technique, the LN cytological study has an essential role prior to molecular analysis. This study aims to verify the lymphatic nature of the sample. This prior morphological examination enabled us to rule out a total of 35 nodes, which were erroneously catalogued as LN at first. The global consistency between cytology and OSNA was 87.4%. Concerning the discrepancies, 93% was due to positive OSNA and negative cytology, mainly when the LNMs were determined as O_mM. There were only five cases considered positive by cytology but negative by OSNA, four of them were ITC according to OSNA. In this last circumstance, the difference is due to a threshold mismatch between a qualitative method (cytology) and a quantitative one (OSNA), in which a minimum metastatic load is required in order to be considered a positive result. The concordance between cytology and OSNA has been analysed by two other groups in smaller series of lymph nodes, reaching similar conclusions.32,33
This work has a series of limitations. OSNA was performed on 57% of the total LNs. Identifying the LNs by palpation of the fresh adipose tissue, an unavoidable initial step for a molecular study, involves a minimum detectable size of the LN. This made it impossible to carry out the OSNA analysis of 429 LNs, resulting in a loss of morphological and molecular information. However, the OSNA analysis was feasible in the majority of the LNMs. We can assume that the data obtained and analysed reflect the information with a probable real clinical impact. The third criterion proposed in order to establish the risk of recurrence related to LNM is the ENE of the metastasis.25,34 LN analysis with OSNA does not allow for a histological study, thereby making it impossible to evaluate this variable. Lastly, we attempted to assess the possible relationship between the quantified tumour load of the LNMs and the variables defined by the guidelines in order to evaluate the risk of recurrence. However, the lack of long-term clinical monitoring does not allow the real prognostic value of the LNMs to be defined. Studies with larger groups of patients and with a longer clinical monitoring period will be necessary to be able to confirm the tendencies highlighted in this work.
In conclusion, we consider that the analysis by OSNA of lymph node metastases from patients with PCT allows a better understanding of the real oncological potential by providing quantitative, objective, and standardised data. In this sense, OSNA could improve efforts to sub-classify the heterogeneous group of N1 patients. Finally, the role of the OSNA assay as part of the clinical management algorithms for these patients still remains to be explored.
Authors’ contributionCI, CZ, OG, SRyC and AGB designed the study; OG and EC performed the surgery; CI and JTS carried out the pathological and molecular processing; CI and CZ collected and analysed the data, performed the literature search and wrote the manuscript; CI, CZ, OG and SRyC interpreted the results; CI, CZ and JTS generated the figures. All authors approve the manuscript, which is not under consideration in any other journal.
Funding sourceThe study was sponsored by Sysmex España S.L.
Conflict of interestNone.