To document current practices in the management of adult patients with hypothyroidism in the setting of primary healthcare.
MethodsWe designed a web-based survey to inquire information on real-life practices regarding management of hypothyroidism by primary care physicians in the region of Madrid (Spain).
ResultsIn total, 546 out of 3897 (14%) physicians (aged 50.9±8.5 yr, 404 females) completed the survey. More than 90% of respondents requested serum thyrotropin measurement in subjects with symptoms of thyroid hypofunction, family history of thyroid disease and history of autoimmune disease. A thyroid ultrasound was requested to evaluate subclinical and overt hypothyroidism by 27.1% and 69.6% of respondents, respectively. Only 22.1% of respondents stated that they do not treat subclinical hypothyroidism with thyrotropin values less than 10mU/l. Most physicians use brand-name formulations of levothyroxine and advise patients on how to take the tablets. To start treatment, the gradual replacement rate was the option chosen by most of the respondents, even in young patients. The thyrotropin target preferred by most respondents was 0.5–5.0mU/l, especially in older patients. In patients with persistent symptoms, 61.4% search for the causes through complementary investigations. A longer professional practice time was not always accompanied by better adherence to guidelines and expert recommendations.
ConclusionOur results reveal a proactive attitude in the diagnosis and of therapy by most of the respondents. However, we observed a tendency to perform unnecessary diagnostic tests and an excessive propensity to treat mild subclinical hypothyroidism.
Documentar la práctica clínica actual en el manejo del hipotiroidismo en adultos.
MétodosSe diseñó una encuesta basada en la web para recabar información sobre las prácticas relativas al manejo del hipotiroidismo por médicos de Atención Primaria de Madrid.
ResultadosQuinientos cuarenta y seis médicos (edad 50,9±8,5 años, 404 mujeres) de un total de 3.897 (14%) completaron la encuesta. Más del 90% solicitaba cuantificación de tirotropina en sujetos con síntomas de hipofunción tiroidea, antecedentes familiares de enfermedad tiroidea e historia de enfermedad autoinmune. El 27,1 y el 69,6% solicitaban ecografía tiroidea para evaluar el hipotiroidismo subclínico y manifiesto, respectivamente. Solo el 22,1% declaró que no trataba el hipotiroidismo subclínico con valores de tirotropina <10mU/l. La mayoría utilizaba preparados de marca de levotiroxina y aconsejaban a los pacientes sobre cómo tomar los comprimidos. La mayoría de los encuestados comenzaba el tratamiento con dosis bajas de levotiroxina, incluso en pacientes jóvenes. El objetivo de tirotropina preferido por la mayoría fue 0,5-5,0mU/l, especialmente en pacientes mayores. En pacientes con síntomas persistentes, el 61,4% buscaba las causas mediante pruebas complementarias. El tiempo de práctica profesional más prolongado no siempre se acompañó de una mejor adherencia a las pautas y recomendaciones de expertos.
ConclusiónNuestros resultados revelan una actitud proactiva de la mayoría de los encuestados en el diagnóstico y el tratamiento del hipotiroidismo. Sin embargo, observamos una tendencia a realizar pruebas diagnósticas innecesarias, así como una propensión excesiva a tratar el hipotiroidismo subclínico leve.
Hypothyroidism is the most common hormonal deficiency worldwide and is managed mainly by primary care physicians (PCPs).1 In a meta-analysis of European studies, the prevalence of undiagnosed hypothyroidism was 4.9%, while the prevalence of known hypothyroidism was clearly lower (3.1%).2 Another study carried out specifically in primary care in Spain3 showed that 8.8% of the general population suffered from hypothyroidism. This frequency was clearly higher in women (13.3%) than in men (4.2%) and raised as the age of the subjects studied increased.
Various international4–6 and national guidelines7 offer recommendations for the screening, diagnosis, and treatment of hypothyroidism. Despite this, many studies have found that 40–50% of patients receiving levothyroxine (L-T4) are poorly controlled due to either under- or overtreatment.8–11
The proper management of this common disease by PCPs is essential to achieve an accurate diagnosis and treatment and avoid comorbidities associated with excess or defect of thyroid hormone. In recent years, several studies dealing with the adherence of patients to replacement treatment and the opinion of patients on their disease, have been reported.12–18 Other investigations have evaluated diagnostic and therapeutic attitudes in primary hypothyroidism by endocrinologists,16,19,20 and two studies have included endocrinologists and general practitioners in France21 and the United States.22 However, we did not find any study investigating this topic in Spain. Therefore, we aimed to document the current practices of PCPs in the management of adult patients with primary hypothyroidism in Madrid (Spain).
MethodsScope of the studyThe scope of this study was all the PCPs (n=3897) working at the Gerencia Asistencial de Atención Primaria (GAAP) of the Community of Madrid (Spain), a public primary care health system that serves a population of 6,784,804 inhabitants.23
Survey designWe worked out a questionnaire to be answered anonymously online. The questionnaire collected information regarding demographic and professional data of participants and their attitude in the detection, diagnosis, treatment, and monitoring of primary hypothyroidism in non-pregnant adults (Supplementary Material, Table S1).
Our survey is an original creation of the authors. The authors designed the questions inquiring about issues commonly encountered in clinical practice, following the natural order of clinical work, that is, screening, diagnosis, treatment, and monitoring. Some questions were based on previously published surveys,16,19–22 and others were newly created. The survey was designed in order that most questions were answered by indicating yes or no. Other questions were multiple-choice, with the possibility of choosing one or more of the answers offered. To avoid bias in multiple-choice questions, we tried to omit phrases that induce the interviewee's answer, and we included a wide range of options ordered alphabetically or randomly. The study was designed to record, but not modify, the usual clinical practice of participants.
Dissemination of the surveyGAAP divides the territory of the Community of Madrid into 7 health areas (South, North, Northwest, East, West, Centre and Southeast). Each of these areas is directed by a Healthcare Management (Dirección Asistencial, DA). All potential interviewees were contacted through the procedures established by GAAP. Thereby, the authors sent the survey information to the directors of the seven DAs in Madrid. An initial mailing was sent immediately before starting the survey (18 September 2019), and a second reminder mailing was sent on 5 November 2019. Each DA was responsible for forwarding the information to the family doctors working at the primary care centres in their area. The survey remained hosted on the web from 18 September to 31 December 2019.
Data collectionParticipants’ responses were collected anonymously and stored electronically in a form hosted on an open-access form creation website (https://www.google.com/forms/).
Statistical analysisResults are expressed as mean±SD for normally distributed data and as median (interquartile range, IQR) for nonparametric data. The Kolmogorov–Smirnov test tested adjustment to normal distribution. For comparisons of means between two groups of subjects, the Student t-test or the Mann–Whitney U-test were used. Categorical variables are described as absolute values and percentages. Because not every participant answered all the questions, the percentage of respondents providing a given answer was calculated individually for each question, using the number of respondents to that question as the denominator. Chi-square tests and Fisher's exact test were used to compare proportions. Differences were considered significant when P<0.05.
ResultsSurveyed physiciansThis web-based survey was responded to by 556 out of 3897 PCPs. Ten registries were excluded because of a lack of information in most questions. The final analysed data set consisted of completed questionnaires from 546 physicians (404 females). The mean age of subjects responding to the survey was 50.9±8.5 years, and the mean time of professional practice was 23.8±8.3 years. Males were older and had a longer time of professional activity than females. The geographical distribution of respondents was as follows: South, 11.4%; North, 12.3%; Northwest, 14.7%; East, 18.1%; West, 11.2%; Centre 12.8%; and Southeast 19.6%. Details on demographic and professional characteristics of participants are provided in Supplementary Material (Table S2).
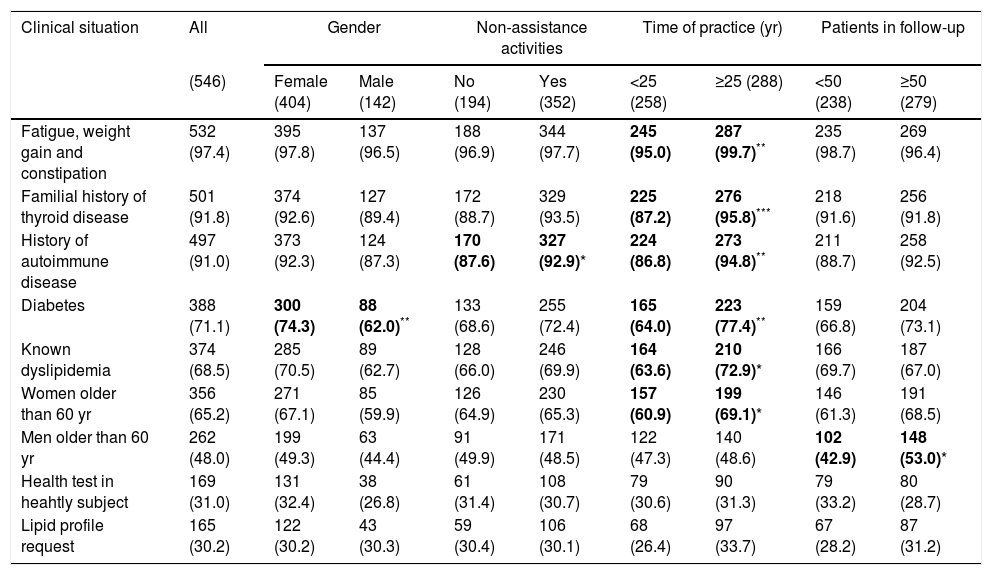
Serum thyrotropin requestThe clinical situations in which the surveyed PCPs request a serum thyrotropin (TSH) measurement as a detection test for hypothyroidism appear in Table 1. Physicians with more than 25 years of professional practice were more likely to screen in some situations, such as women older than 60 years (P<0.05), history of autoimmune disease (P<0.01), dyslipidemia (P<0.05), diabetes (P<0.01), family history of thyroid disease (P<0.001) and symptoms (P<0.01) (Table 1).
Clinical situations in which surveyed primary care physicians request serum thyrotropin determination.
| Clinical situation | All | Gender | Non-assistance activities | Time of practice (yr) | Patients in follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|
| (546) | Female (404) | Male (142) | No (194) | Yes (352) | <25 (258) | ≥25 (288) | <50 (238) | ≥50 (279) | |
| Fatigue, weight gain and constipation | 532 (97.4) | 395 (97.8) | 137 (96.5) | 188 (96.9) | 344 (97.7) | 245 (95.0) | 287 (99.7)** | 235 (98.7) | 269 (96.4) |
| Familial history of thyroid disease | 501 (91.8) | 374 (92.6) | 127 (89.4) | 172 (88.7) | 329 (93.5) | 225 (87.2) | 276 (95.8)*** | 218 (91.6) | 256 (91.8) |
| History of autoimmune disease | 497 (91.0) | 373 (92.3) | 124 (87.3) | 170 (87.6) | 327 (92.9)* | 224 (86.8) | 273 (94.8)** | 211 (88.7) | 258 (92.5) |
| Diabetes | 388 (71.1) | 300 (74.3) | 88 (62.0)** | 133 (68.6) | 255 (72.4) | 165 (64.0) | 223 (77.4)** | 159 (66.8) | 204 (73.1) |
| Known dyslipidemia | 374 (68.5) | 285 (70.5) | 89 (62.7) | 128 (66.0) | 246 (69.9) | 164 (63.6) | 210 (72.9)* | 166 (69.7) | 187 (67.0) |
| Women older than 60 yr | 356 (65.2) | 271 (67.1) | 85 (59.9) | 126 (64.9) | 230 (65.3) | 157 (60.9) | 199 (69.1)* | 146 (61.3) | 191 (68.5) |
| Men older than 60 yr | 262 (48.0) | 199 (49.3) | 63 (44.4) | 91 (49.9) | 171 (48.5) | 122 (47.3) | 140 (48.6) | 102 (42.9) | 148 (53.0)* |
| Health test in heahtly subject | 169 (31.0) | 131 (32.4) | 38 (26.8) | 61 (31.4) | 108 (30.7) | 79 (30.6) | 90 (31.3) | 79 (33.2) | 80 (28.7) |
| Lipid profile request | 165 (30.2) | 122 (30.2) | 43 (30.3) | 59 (30.4) | 106 (30.1) | 68 (26.4) | 97 (33.7) | 67 (28.2) | 87 (31.2) |
Data are the number (percentage) of affirmative answers for each of the situations in which the respondents’ opinion is requested about the screening.
Figures in parentheses in the headings of each column indicate the number of subjects in each group or subgroup.
Statistically significant differences (chi-square test) are highlighted in bold.
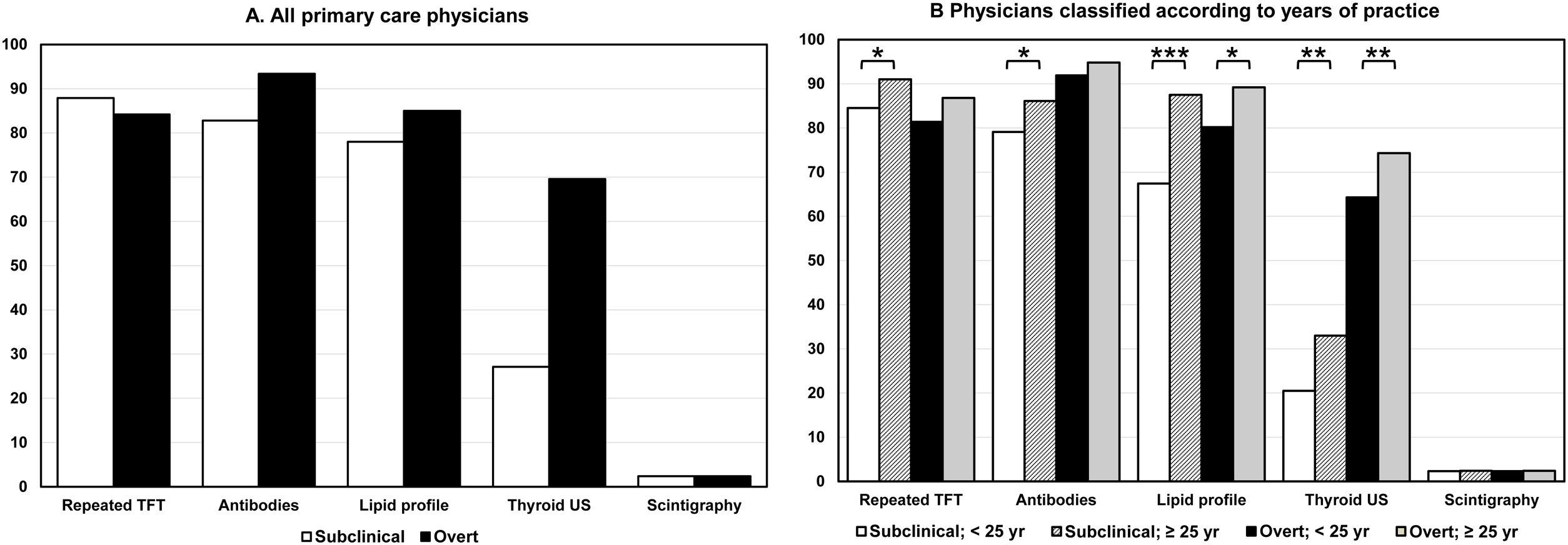
In the diagnostic evaluation of patients with biochemical evidence of hypothyroidism, most respondents requested the second quantification of thyroid function tests (TFT), i.e., TSH and free thyroxine (FT4), and thyroid antibodies and lipid profile measurement. Thyroid ultrasound (US) was requested for the diagnostic evaluation of subclinical hypothyroidism (SH) and overt hypothyroidism (OH) by 27.1% and 69.6% of the respondents, respectively (Fig. 1A).
Affirmative responses (percentage) about the request for complementary investigations after the biochemical diagnosis of subclinical (open columns) and overt hypothyroidism (closed columns) in adult patients. (A) All surveyed physicians. (B) Physicians classified according to years of professional practice. Abscissa scale: percentage of affirmative responses. Abbreviations: TFT, thyroid function tests; US, ultrasound. *P<0.05; **P<0.01; ***P<0.001.
Again, we found few differences in the respondents classified by gender, extra-healthcare activity and the number of patients under follow-up (Supplementary Material, Table S3). However, physicians with more than 25 years of practice were more likely to request a second determination of TFT, antibodies, lipid profile and US in patients with SH and lipid profile and US in patients with OH (Fig. 1B).
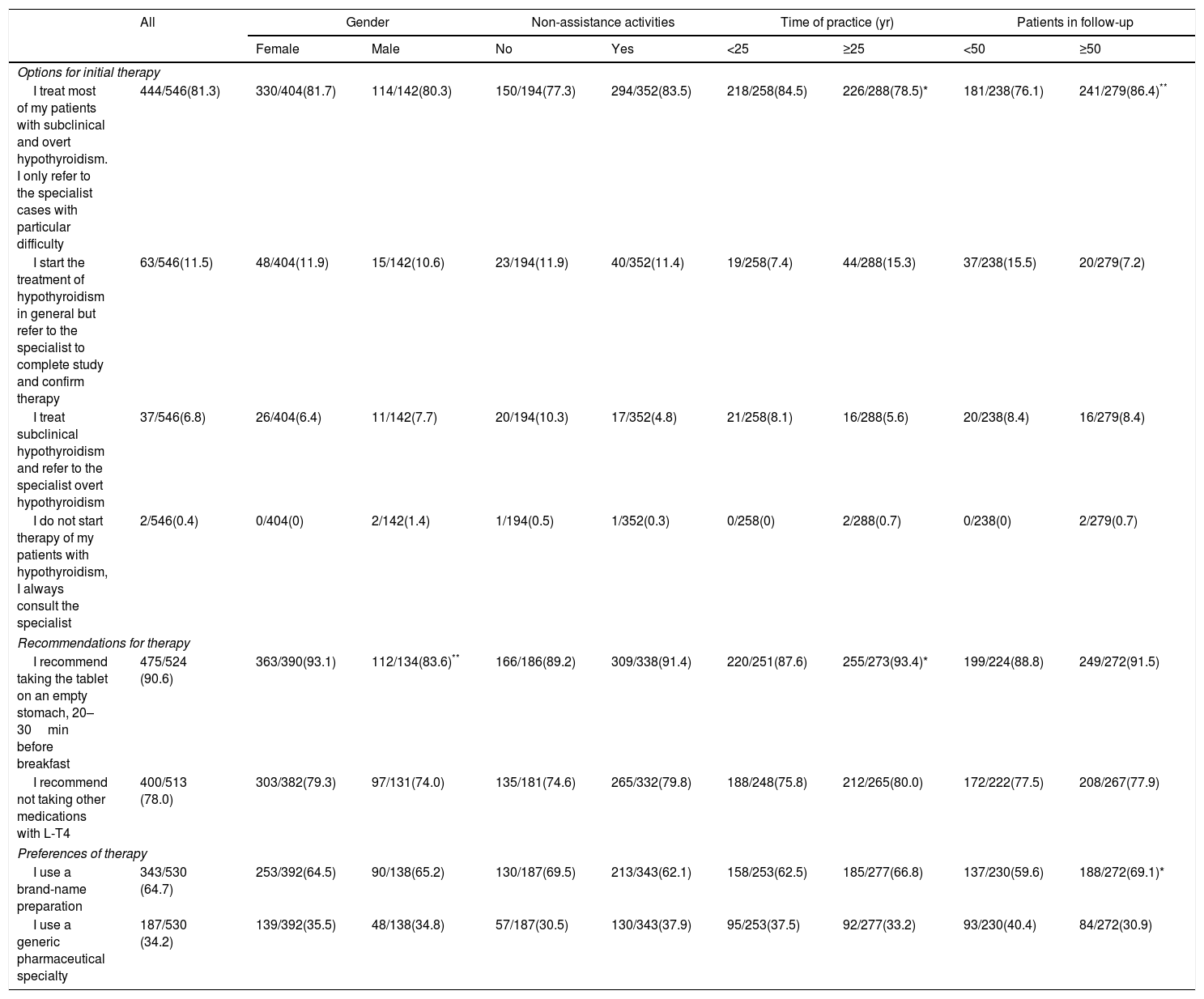
TreatmentMost respondents (81.3%) stated that they treat most of their patients with SH or OH. Only 2 respondents (0.4%) do not start treatment and send their patients to the specialist (Table 2). Physicians with more than 50 patients on follow-up were more proactive in treating most of their patients. However, physicians with more than 25 years of practice were less proactive than those with fewer years of experience (78.5 vs 84.5%, P<0.05).
Attitude of primary care practitioners in establishing the initial therapy in patients with hypothyroidism.
| All | Gender | Non-assistance activities | Time of practice (yr) | Patients in follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | No | Yes | <25 | ≥25 | <50 | ≥50 | ||
| Options for initial therapy | |||||||||
| I treat most of my patients with subclinical and overt hypothyroidism. I only refer to the specialist cases with particular difficulty | 444/546(81.3) | 330/404(81.7) | 114/142(80.3) | 150/194(77.3) | 294/352(83.5) | 218/258(84.5) | 226/288(78.5)* | 181/238(76.1) | 241/279(86.4)** |
| I start the treatment of hypothyroidism in general but refer to the specialist to complete study and confirm therapy | 63/546(11.5) | 48/404(11.9) | 15/142(10.6) | 23/194(11.9) | 40/352(11.4) | 19/258(7.4) | 44/288(15.3) | 37/238(15.5) | 20/279(7.2) |
| I treat subclinical hypothyroidism and refer to the specialist overt hypothyroidism | 37/546(6.8) | 26/404(6.4) | 11/142(7.7) | 20/194(10.3) | 17/352(4.8) | 21/258(8.1) | 16/288(5.6) | 20/238(8.4) | 16/279(8.4) |
| I do not start therapy of my patients with hypothyroidism, I always consult the specialist | 2/546(0.4) | 0/404(0) | 2/142(1.4) | 1/194(0.5) | 1/352(0.3) | 0/258(0) | 2/288(0.7) | 0/238(0) | 2/279(0.7) |
| Recommendations for therapy | |||||||||
| I recommend taking the tablet on an empty stomach, 20–30min before breakfast | 475/524 (90.6) | 363/390(93.1) | 112/134(83.6)** | 166/186(89.2) | 309/338(91.4) | 220/251(87.6) | 255/273(93.4)* | 199/224(88.8) | 249/272(91.5) |
| I recommend not taking other medications with L-T4 | 400/513 (78.0) | 303/382(79.3) | 97/131(74.0) | 135/181(74.6) | 265/332(79.8) | 188/248(75.8) | 212/265(80.0) | 172/222(77.5) | 208/267(77.9) |
| Preferences of therapy | |||||||||
| I use a brand-name preparation | 343/530 (64.7) | 253/392(64.5) | 90/138(65.2) | 130/187(69.5) | 213/343(62.1) | 158/253(62.5) | 185/277(66.8) | 137/230(59.6) | 188/272(69.1)* |
| I use a generic pharmaceutical specialty | 187/530 (34.2) | 139/392(35.5) | 48/138(34.8) | 57/187(30.5) | 130/343(37.9) | 95/253(37.5) | 92/277(33.2) | 93/230(40.4) | 84/272(30.9) |
Data are the number of affirmative responses for each item/number of valid responses (percentage).
22.1% of respondents stated that SH with TSH values between 5 and 10mU/l (mild SH) should not be treated. The criteria for L-T4 treatment of patients with mild SH were symptoms (57.7% of respondents), antibodies (32.0%), and goitre (22.7%). On the contrary, only 0.7% of respondents believed SH with TSH values >10mU/l should not be treated. The criteria for treatment of these patients were symptoms (32.7%), antibodies (25.6%) and goitre (18.4%).
Most of the respondents recommended taking L-T4 tablets on an empty stomach (90.6%) and avoiding taking other medications (78.0%) (Table 3). Approximately two-thirds of physicians preferred to use a brand-name preparation. We found few significant differences in the recommendations for taking tablets and in the preferences of pharmaceutical formulations in the practitioners classified in the groups that appear in Table 3.
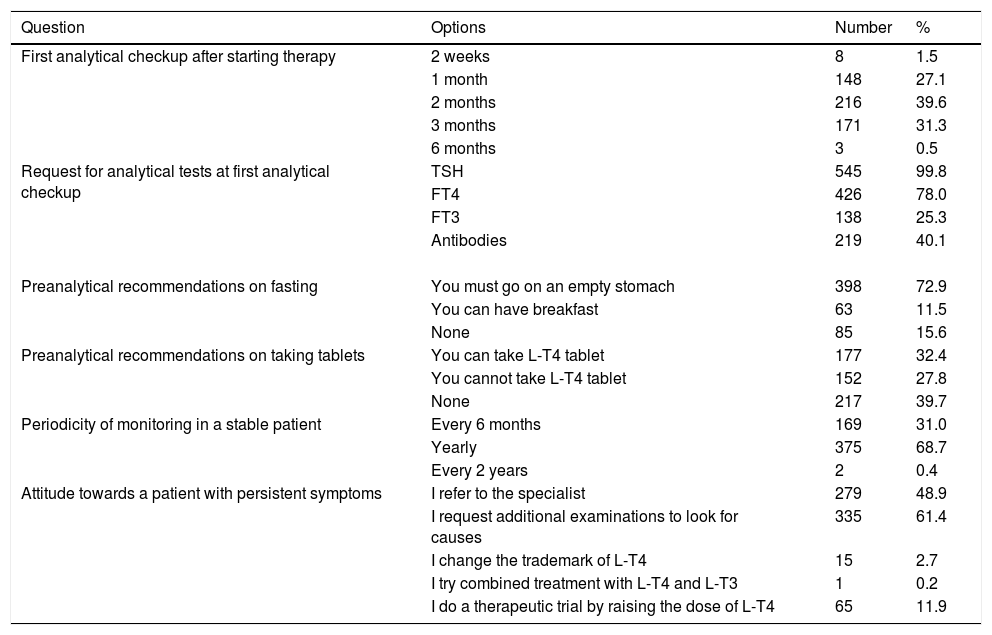
Monitoring of therapy and follow-up of patients with hypothyroidism.
| Question | Options | Number | % |
|---|---|---|---|
| First analytical checkup after starting therapy | 2 weeks | 8 | 1.5 |
| 1 month | 148 | 27.1 | |
| 2 months | 216 | 39.6 | |
| 3 months | 171 | 31.3 | |
| 6 months | 3 | 0.5 | |
| Request for analytical tests at first analytical checkup | TSH | 545 | 99.8 |
| FT4 | 426 | 78.0 | |
| FT3 | 138 | 25.3 | |
| Antibodies | 219 | 40.1 | |
| Preanalytical recommendations on fasting | You must go on an empty stomach | 398 | 72.9 |
| You can have breakfast | 63 | 11.5 | |
| None | 85 | 15.6 | |
| Preanalytical recommendations on taking tablets | You can take L-T4 tablet | 177 | 32.4 |
| You cannot take L-T4 tablet | 152 | 27.8 | |
| None | 217 | 39.7 | |
| Periodicity of monitoring in a stable patient | Every 6 months | 169 | 31.0 |
| Yearly | 375 | 68.7 | |
| Every 2 years | 2 | 0.4 | |
| Attitude towards a patient with persistent symptoms | I refer to the specialist | 279 | 48.9 |
| I request additional examinations to look for causes | 335 | 61.4 | |
| I change the trademark of L-T4 | 15 | 2.7 | |
| I try combined treatment with L-T4 and L-T3 | 1 | 0.2 | |
| I do a therapeutic trial by raising the dose of L-T4 | 65 | 11.9 |
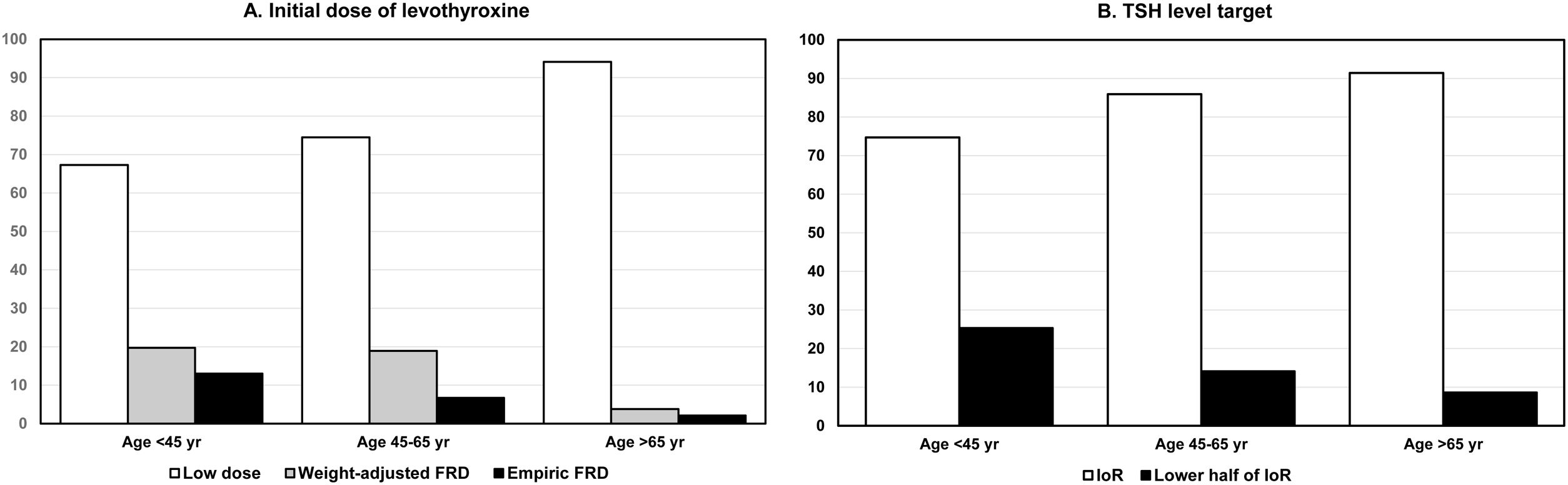
The most preferred option to start treating hypothyroidism was to use low doses of L-T4 with progressive increase according to TSH response, even in patients <45 years. This option was more used as the patient's age increased. Weight-adjusted or empirical full replacement dose (FRD) was used in patients under 45 years of age by 19.7% and 13.0% of the respondents, respectively. In patients aged 45 to 60, these percentages were 18.9% and 6.7%, respectively (Fig. 2A). The TSH target preferred by most respondents was 0.5–5.0mU/l, that is, the interval of reference (IoR). This opinion became more prevalent as the patient's age increased (Fig. 2B).
(A) Initial dose for starting therapy of overt hypothyroidism according to the age of patients: low dose with progressive rise according to TSH response (open columns), weight-adjusted full replacement dose (grey columns), and empiric full replacement dose (closed columns). (B) Target of serum TSH in patients classified in the same age groups: interval of reference (0.5–5.0mU/l) (open columns), and lower half of interval of reference (0.5–2.5mU/ml) (closed columns). Abscissa scale: percentage of affirmative responses. Abbreviations: FRD, full replacement dose; IoR, interval of reference.
Table 3 shows the preferences of the respondents on monitoring and follow-up. After starting therapy, the first reevaluation of TFT was carried out between 1 and 3 months by 98.0% of the respondents. Most physicians (68.7%) perform an annual TSH measurement in stable patients. In patients with persistent symptoms, 61.4% search for the causes through complementary investigations, but 48.9% refer them to the specialist in Endocrinology.
Influence of professional experience and volume of patientsTable 4 shows the differences in some selected criteria for detection, diagnosis, treatment, and monitoring related to professional experience, evaluated by years of practice and number of patients under follow-up. The number of patients under follow-up was higher in physicians who decided to treat most of their hypothyroid patients, compared to those who did not (50 [30–90] vs 30 [20–60]; P<0.01).
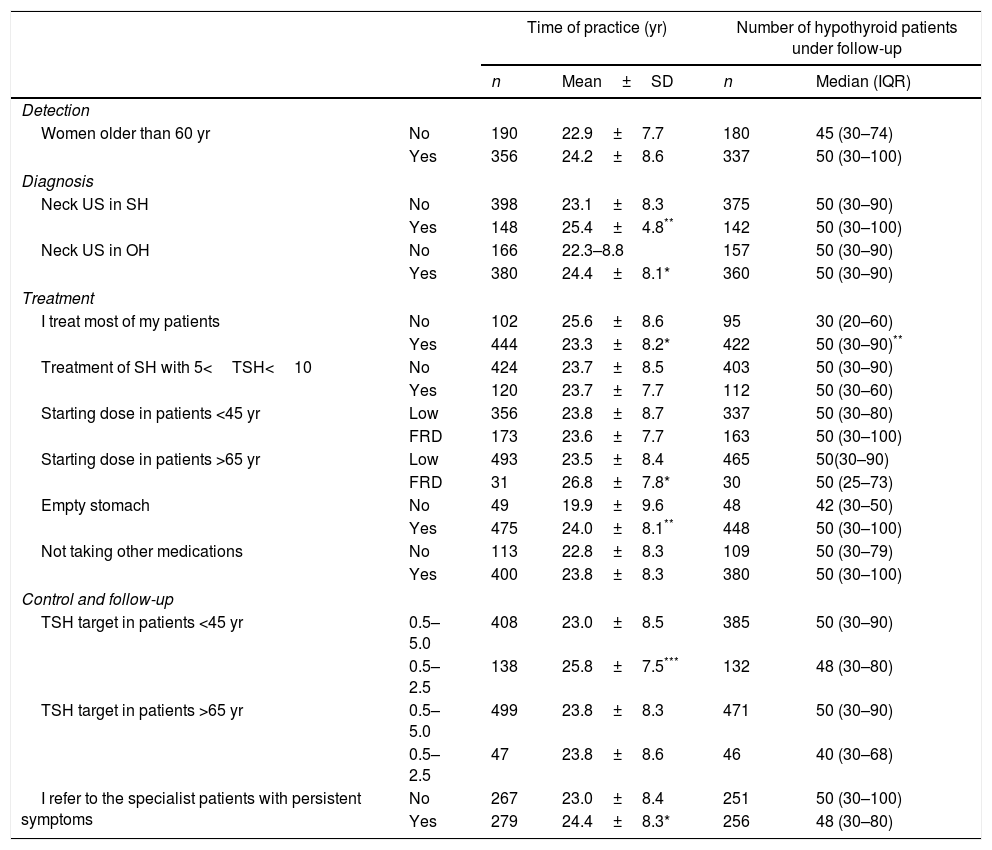
Relationships between professional experience (years of practice and the number of hypothyroid patients under follow-up) with some criteria for detection, diagnosis, treatment and follow-up of hypothyroidism.
| Time of practice (yr) | Number of hypothyroid patients under follow-up | ||||
|---|---|---|---|---|---|
| n | Mean±SD | n | Median (IQR) | ||
| Detection | |||||
| Women older than 60 yr | No | 190 | 22.9±7.7 | 180 | 45 (30–74) |
| Yes | 356 | 24.2±8.6 | 337 | 50 (30–100) | |
| Diagnosis | |||||
| Neck US in SH | No | 398 | 23.1±8.3 | 375 | 50 (30–90) |
| Yes | 148 | 25.4±4.8** | 142 | 50 (30–100) | |
| Neck US in OH | No | 166 | 22.3–8.8 | 157 | 50 (30–90) |
| Yes | 380 | 24.4±8.1* | 360 | 50 (30–90) | |
| Treatment | |||||
| I treat most of my patients | No | 102 | 25.6±8.6 | 95 | 30 (20–60) |
| Yes | 444 | 23.3±8.2* | 422 | 50 (30–90)** | |
| Treatment of SH with 5<TSH<10 | No | 424 | 23.7±8.5 | 403 | 50 (30–90) |
| Yes | 120 | 23.7±7.7 | 112 | 50 (30–60) | |
| Starting dose in patients <45 yr | Low | 356 | 23.8±8.7 | 337 | 50 (30–80) |
| FRD | 173 | 23.6±7.7 | 163 | 50 (30–100) | |
| Starting dose in patients >65 yr | Low | 493 | 23.5±8.4 | 465 | 50(30–90) |
| FRD | 31 | 26.8±7.8* | 30 | 50 (25–73) | |
| Empty stomach | No | 49 | 19.9±9.6 | 48 | 42 (30–50) |
| Yes | 475 | 24.0±8.1** | 448 | 50 (30–100) | |
| Not taking other medications | No | 113 | 22.8±8.3 | 109 | 50 (30–79) |
| Yes | 400 | 23.8±8.3 | 380 | 50 (30–100) | |
| Control and follow-up | |||||
| TSH target in patients <45 yr | 0.5–5.0 | 408 | 23.0±8.5 | 385 | 50 (30–90) |
| 0.5–2.5 | 138 | 25.8±7.5*** | 132 | 48 (30–80) | |
| TSH target in patients >65 yr | 0.5–5.0 | 499 | 23.8±8.3 | 471 | 50 (30–90) |
| 0.5–2.5 | 47 | 23.8±8.6 | 46 | 40 (30–68) | |
| I refer to the specialist patients with persistent symptoms | No | 267 | 23.0±8.4 | 251 | 50 (30–100) |
| Yes | 279 | 24.4±8.3* | 256 | 48 (30–80) | |
Abbreviations: SH, subclinical hypothyroidism; OH, overt hypothyroidism; US, ultrasound; FRD, full replacement dose.
Time of professional practice was related to some of these criteria. Physicians who requested neck US to evaluate patients with SH or OH had significantly longer professional practice than those who did not. Furthermore, more extended time of practice was also observed in the options of choosing FRD in patients >65 years and referring patients with persistent symptoms to the specialist. On the contrary, doctors with more extended professional experience were more proactive in treating most of their patients and were more likely to select a TSH target in the lower half of the IoR in patients younger than 45 years.
DiscussionThyroid dysfunction screening by measurement of serum TSH is a matter of international debate.24–26 Our survey results show that Madrid's PCPs show a high level of awareness of hypothyroidism and agree with these general recommendations. Interestingly, some societies recommend screening in subjects over 50–60 years of age, especially in women.26,27 However, only 48.0% and 65.2% of the respondents agreed with screening men and women, respectively, over 60 years of age. It is noteworthy that 71.1% of PCPs were favourable to detecting hypothyroidism in diabetic patients, as has been proposed by some authors.28–30
Most of the respondents requested a second TFT determination, antibody testing, and lipid panel in both SH and OH diagnostic evaluation. Thyroid US is not recommended routinely in hypothyroid patients in current guidelines.5,31 Nonetheless, it is striking that 27.1% of respondents requested this study in patients with SH and 69.6% in patients with OH. In addition, those with more than 25 years of practice are more likely to request thyroid US. This may reflect an increased presence of US devices in primary care centres, or a greater interest of the PCPs to evaluate the presence of thyroid nodules or Hashimoto's thyroiditis in these patients.
Patients with SH and TSH values in the 5–10mU/l range represent a subset in which treatment with thyroid hormone is controversial.5 Remarkably, more than 50% of respondents treat mild SH (TSH levels 5–10mU/l) because of the presence of symptoms with the expectation that symptoms may improve. Many physicians also use the presence of antibodies to decide the treatment of SH, but guidelines only recommend using thyroid antibodies for investigating the cause of hypothyroidism and not for the decision to start treatment.5
In general, we observed a tendency to overtreatment of SH since only 22.1% of respondents stated that they do not treat SH with TSH of 5–10mU/l. This agrees with a trend toward a lower threshold for treating SH reported in United Kingdom32 and Denmark.33 However, this overtreatment might cause a significant proportion of patients treated with thyroid hormone to be in a situation of subclinical thyrotoxicosis, as has been shown by some studies.8–10,34,35
Most PCPs offer appropriate advice to their patients on how to take the tablets. Brand name preparations of L-T4 was preferred by 64.7% of PCPs, in contrast to 49.9% of endocrinologists in the survey by Burch et al.19 and to 83% of ATA members in the survey by McDermott et al.22 Starting therapy with FRD is safe in otherwise healthy individuals.22,36 However, the gradual rate of replacement, starting with low doses, was the option chosen by 67.3–94.2% of PCPs, according to the age of the patients. This gradual rate of replacement has been reported in half of the patients studied by Delemer et al.,21 and was the option chosen by 38.5% of endocrinologists in the survey of Burch et al.19 Starting therapy with FRD in young patients was more common among specialists than among PCPs in the survey of McDermott et al.22 Our survey did not include any questions about the use of different L-T4 formulations, such as liquid solution or soft-gel capsules, as the only L-T4 available in Spain is in tablet form.
Contrary to the opinion of endocrinologists in previous studies,19,22 a target of TSH in the lower half of IoR was an option scarcely chosen by our respondents, even in young patients (25.3% in patients <45 years). As previously shown,22 PCPs often chose a broader TSH goal in patients of all ages, and it seems that they are not comfortable with a TSH target in the lower half of the IoR.
Rechecking TFT after starting therapy was performed between 1 and 3 months by 98.0% of respondents. It is striking that, apart from serum TSH, many respondents also request determinations not recommended by guidelines (FT4) or not helpful in monitoring (FT3 and antibodies).5 After achieving stable TSH values, 68.7% of respondents obtained laboratory studies yearly and 31.0% at 6-months intervals. In contrast, 34.0% and 55.5% of endocrinologists performed monitoring every 12 and 6 months, respectively.19 Although some studies have shown a transient suppression of serum TSH levels after L-T4 dosing,37 only 27.8% of respondents recommended blood sampling for TSH before L-T4 ingestion.
Symptoms of hypothyroidism are not specific, and each of the symptoms generally associated with hypothyroidism may also have non-thyroid causes.38 In our survey, 61.4% of respondents performed testing for other sources of the patient's symptoms, in contrast to 84.3% of endocrinologists.19 It is noticeable that 48.9% of family doctors refer these patients to the specialist. As recommended by guidelines4,6 the use of T3-containing therapies was anecdotic.
Our data suggest that a longer professional practice time is not always accompanied by better adherence to guideline recommendations. This could be the case for the use of US in diagnosis and the preference for FRD in patients older than 65 years. Surprisingly, PCPs less prone to treat their patients and more favourable to refer to the specialist patients with persistent symptoms have more professional practice time.
We believe our results are likely to represent current practices in primary care in Madrid since the survey was answered by 14% of PCPs. This percentage is in line with that obtained in other studies on hypothyroidism. In the survey by McDermott et al.,22 the response rate was 24% of the invited PCPs. In the study by Delemer et al.,21 9.5% of the PCPs agreed to participate. In the report by Burch et al.,19 10.9% of the members of the Endocrine Society responded to the survey. Moreover, the distribution by geographic areas of the responding PCPs from Madrid was regular, and there was no health area with less than 10% of respondents.
Furthermore, most of the respondents answered all the questions on the survey, and there were very few unanswered questions. The study was limited to Madrid; therefore, results cannot be extrapolated to other geographical regions. It is possible that PCPs who are more interested in hypothyroidism may have been more willing to respond to the survey than other physicians. In addition, results may be biased by this fact, that is, the management of hypothyroidism by such a group of dedicated doctors could differ significantly from that of the average physicians. To settle this point, a new study may be needed to eliminate any doubt about the existence of such bias. The relationship and communication of PCPs with the Endocrinology departments of Madrid's different health areas was not analysed in this study. It is possible that these relationships are different in the various areas and that, therefore, this may condition differences in the management of hypothyroidism.
In summary, the present report shows that the management of hypothyroidism by the Madrid PCPs broadly follow the recommendations of the international guidelines. However, PCPs tend to deviate from the recommendations due to the widespread use of thyroid US and thyroid antibodies, the criteria for starting therapy of SH and the definition of TSH targets according to age.
Data availabilityThe data are available from the corresponding author upon request.
Ethical approvalThe study received favourable reports from the Research Ethics Committee of the Hospital Universitario Puerta de Hierro Majadahonda and the Central Research Committee of the GAAP (code 37/19).
FundingNone.
Conflict of interestNone.
We acknowledge the directors of the seven DAs of Madrid who assisted with disseminating our survey and the PCPs who took the time to complete this survey.