Inferior petrosal sinus sampling (IPSS) is indicated in the diagnosis of adrenocorticotropic hormone (ACTH)-dependent Cushing's syndrome (CS), especially when the results of the initial diagnostic tests are discordant.
ObjectiveTo describe the patients who underwent this invasive functional test in a tertiary hospital.
MethodsThis was an observational study of a retrospective cohort of patients with ACTH-dependent CS and IPSS between 2004 and 2019. We determined their epidemiological, hormonal, radiological and functional characteristics, and evaluated their diagnostic capacity and optimal cut-off points to differentiate between Cushing's disease (CD) and ectopic Cushing's syndrome (ECS).
Results23 patients were evaluated, of which 65.2% were women with the average age of 42 (36–62) years. ACTH secretion of pituitary origin was evident in 82.6% of the patients and of ectopic origin in 17.4%. Plasma cortisol, urinary free cortisol, and ACTH levels were higher in patients with ECS. Regarding IPSS, the baseline central/peripheral ACTH gradient detected 89.5% of patients with CD and after stimulation with CRH, 100%. The optimal cut-off points in the diagnosis of CD were 2.06 at baseline and 2.49 after CRH stimulation.
ConclusionIPSS with CRH stimulation is a test with a high diagnostic accuracy for correctly classifying patients with CD and ECS. The cut-off points of the gradients may be different from the classic ones. Therefore, we recommend that each center perform its own evaluation.
El cateterismo de senos petrosos inferiores (CSPI) está indicado en el diagnóstico de síndrome de Cushing (SC) hormona adrenocorticotropa (ACTH) dependiente, especialmente cuando los resultados del resto de las pruebas diagnósticas son discordantes.
ObjetivoDescribir los pacientes a quienes se les realizó esta prueba invasiva funcional en un hospital de tercer nivel.
MétodosEstudio observacional sobre una cohorte retrospectiva de pacientes con SC ACTH-dependiente y CSPI entre los años 2004 y 2019. Se determinaron sus características epidemiológicas, hormonales, radiológicas y funcionales, evaluándose su capacidad diagnóstica y puntos de corte óptimos para diferenciar entre enfermedad de Cushing (EC) y síndrome de Cushing ectópico (SCE).
ResultadosFueron evaluados 23 pacientes de los cuales 65,2% fueron mujeres y la edad promedio fue 42 (36–62) años. Se evidenció una secreción de ACTH de origen hipofisario en el 82,6% de los pacientes y de origen ectópico en el 17,4%. Los niveles de cortisol plasmático, cortisol libre urinario y ACTH fueron más elevados en pacientes con SCE. Respecto al CSPI, el gradiente ACTH central/periférico (C/P) basal detectó el 89,5% de pacientes con EC y tras estimulación con de la hormona liberadora de corticotropina (CRH) el 100%, siendo los puntos de corte óptimos para el diagnóstico de EC 2,06 basal y 2,49 tras estimulación con CRH.
ConclusionesEl CSPI con estimulación de CRH es una prueba con alta precisión diagnóstica para clasificar de manera correcta a los pacientes con EC y SCE. Los puntos de corte de los gradientes pueden ser distintos a los valores clásicos, por lo que recomendamos que cada centro los evalúe.
Cushing's syndrome (CS) comprises a set of signs and symptoms that are due to chronic exposure to high concentrations of glucocorticoids. It is a potentially fatal disorder due to the comorbidities it causes: arterial hypertension, diabetes mellitus, coagulopathy, cardiovascular disease, infections and fractures, to name a few. Therefore, a delayed diagnosis can substantially increase the mortality rate.1
CS can develop dependently or independently of adrenocorticotropic hormone (ACTH). ACTH-dependent forms account for around 80–85% of cases and are characterised by excessive ACTH production, usually from a pituitary adenoma (Cushing's disease [CD]) or more rarely from an extra-pituitary tumour (ectopic Cushing's syndrome [ECS]).2 Differentiating between the two causes can be complex, especially when the laboratory results or functional tests are discordant or lesions are not visible on pituitary magnetic resonance imaging (PMRI).3,4 In these situations, conducting an invasive functional test is required. Inferior petrosal sinus sampling (IPSS) has been used since 1970 and is currently considered the gold standard to determine whether ACTH secretion is central or ectopic, as well as its lateralisation in the case of CD.5,6 In order to increase the diagnostic sensitivity of the procedure, it is usually performed with corticotropin-releasing hormone (CRH) stimulation.7
The primary endpoint of this study was to evaluate the diagnostic accuracy offered by the CRH stimulation test and IPSS, and to construct receiver operating characteristic (ROC) curves to see whether the cut-off points established in previous studies with respect to the central to peripheral (C:P) ACTH gradient resemble those found in the experience of our centre. As secondary endpoints, we analysed the different epidemiological, hormonal and functional parameters found in the patients from our centre with ACTH-dependent CS and IPSS performed, and examined the clinical differences between patients with CD and those with ECS.
Material and methodsStudy design and sample selectionAn observational study was conducted on a retrospective cohort of patients diagnosed with ACTH-dependent CS between 2004 and 2019, at the Endocrinology and Nutrition Department of the Hospital General Universitario Gregorio Marañón [Gregorio Marañón General University Hospital], who underwent inferior petrosal sinus sampling.
Diagnostic approach to Cushing's syndromeThe initial diagnosis of CS was made by conducting, on at least two occasions, a 24-h urinary free cortisol test (UFC), a suppression test with 1 mg of dexamethasone at night and/or salivary cortisol at 23 h.2 Those patients with pseudo-Cushing's states were excluded.
Regarding the aetiological diagnosis of CS, ACTH was determined and those cases with values >15 pg/mL were considered indicative of ACTH-dependent CS (pituitary or ectopic). Regarding the CRH stimulation test, an increase in ACTH > 50% or serum cortisol >20% over the baseline value was considered indicative of CD.8 A PMRI was also performed in all patients.
IPSS was performed on all patients, mainly due to dubious imaging or functional tests. Those that did not have the aforementioned studies or who were cases of ACTH-independent CS were excluded from the statistical analysis.
The procedure was carried out at the Radiology Department by an experienced team consisting of two interventional neuroradiologists, an anaesthetist, an endocrinologist and a nurse. The same IPSS technique was used in all patients. The test consisted of cannulating both antecubital veins (one of them for blood draw and the other for stimulation injection) as well as catheterising the two femoral veins until the inferior petrosal sinuses were reached. The location of the two catheters was verified radiologically. Blood samples were then taken from a peripheral vein and from each of the inferior petrosal sinuses simultaneously at the start of the study and after an intravenous bolus injection of 100 µg of CRH. Post-stimulation samples were obtained at 3, 5, 10 and 15 min. The procedure was completed by obtaining cortisol and ACTH peripheral blood samples at 30, 60, 90 and 120 min after CRH stimulation. The peak post-stimulation cortisol and ACTH values obtained were taken into consideration. All the samples for ACTH testing were collected in tubes containing ethylenediaminetetraacetic acid (EDTA) and immediately refrigerated and transported to our laboratory. After the procedure, the patients remained in bed and were kept under observation for six hours. Any periprocedural complications were recorded.
An ACTH or C:P gradient ≥2 in baseline samples or ≥3 in samples obtained after stimulation with CRH was established as a reference value indicative of CD.8,9 In addition, an interpetrosal gradient ≥1.4 was taken into account as suggestive of an adenoma location on the side of the petrosal sinus with the highest concentration of ACTH.9,10 To determine the final aetiological diagnosis of CS, the results of the CRH stimulation test, IPSS, PMRI, histology and immunohistochemistry, as well as biochemical criteria for recovery after surgery were taken into account. Criteria for recovery were morning serum cortisol <5 mg/dl or normal 24-h UFC excretion.
Statistical analysisThe distribution of quantitative variables was studied with the Shapiro–Wilk test, showing a non-normal distribution and therefore being expressed as medians and interquartile ranges (p25-p75). The Mann–Whitney U test was used to compare two independent sample medians. The qualitative variables were expressed in absolute and relative frequencies (percentages). The X2 test was used to study associations between qualitative variables, and, if necessary, Fisher's correction was applied.
ROC curves were used to determine the area under the curve (AUC) of: (a) increase in ACTH (⊗ACTH) after CRH stimulation, (b) baseline C:P ACTH gradient, (c) C:P ACTH gradient after CRH stimulation for the diagnosis of CD, evaluating the sensitivity and specificity of the cut-off points. To select the best cut-off point, the distance to the point closest to 0.1 was used.
p < 0.05 was considered statistically significant. For descriptive and inferential analysis, the SPSS version 22 statistical package was used.
ResultsClinical characteristics of the patientsIn total, 23 patients with a confirmed diagnosis of ACTH-dependent CS who underwent IPSS were evaluated.
The median age at diagnosis of CD was 44.0 (37.0–62.0) years, and in ECS, 35.5 (30.0–51.0) years, without statistically significant differences. Overall, 65.2% (n = 15) of the patients were female, with 73.7% of CD cases (n = 14) arising in women, while most ECS cases were in men (75.0%, n = 3), without statistically significant differences.
Of the 23 cases included, the IPSS results identified pituitary ACTH secretion in 19 patients (82.6%) and ectopic secretion in four (17.4%). The diagnosis of CD was confirmed by positive immunohistochemistry for ACTH in 14 of 19 patients. In the five remaining patients, diagnosis of CD was assumed by biochemical recovery criteria after pituitary surgery/radiotherapy and/or by concordant diagnostic tests (response to the CRH test, identification of the tumour on PMRI and C:P ACTH gradient).8 Regarding the cases of ECS, all of them were diagnosed with lung carcinoid tumours, which were located by imaging tests (full body computed tomography, somatostatin receptor scintigraphy and/or positron emission tomography-computed tomography) and referred for biopsy or excision, with the final diagnosis being histologically confirmed in all of them.
Regarding the complications observed during and after IPSS, adverse events were only reported in two cases: earache in the first and low-risk pulmonary thromboembolism in the second.
The biochemical tests revealed higher serum cortisol, urinary free cortisol and ACTH levels in patients with ECS than in those with CD. The CRH stimulation test showed a higher average increase in cortisol and ACTH in patients with CD (p < 0.01), which facilitated the identification of 18 of the 19 patients with pituitary Cushing's (Table 1).
Biochemical and functional tests in patients with ACTH-dependent Cushing's Syndrome and IPSS performed. Hospital General Universitario Gregorio Marañón 2004-2019.
| CD | ECS | ||||||
|---|---|---|---|---|---|---|---|
| Median | p25 | p75 | Median | p25 | p75 | p* | |
| SC (μg/dl) | 23.60 | 16.70 | 36.70 | 44.75 | 36.80 | 52.10 | 0.003 |
| UFC (μg/24 h) | 314 | 194 | 1080 | 2,164.50 | 1068 | 6246 | 0.002 |
| ACTH (pg/mL) | 41.40 | 28.60 | 200 | 183.90 | 110.40 | 263 | 0.003 |
| ΔSC after CRH (%)a | 69.40 | 40 | 200 | 10.17 | 8.83 | 11.50 | 0.019 |
| ΔACTH after CRH(%)a | 119.42 | 63.35 | 969.80 | 32.22 | 10.21 | 87.57 | 0.009 |
| Baseline C:P ACTH gradientb | 4.96 | 3.23 | 30.89 | 1.39 | 1.21 | 1.78 | 0.006 |
| C:P ACTH gradient after CRHb | 26.10 | 8.44 | 225.50 | 1.51 | 1.43 | 1.73 | <0.001 |
| Interpetrosal ACTH gradientc | 3.25 | 2.04 | 24.11 | 1.18 | 1.11 | 1.47 | <0.001 |
ACTH: adrenocorticotropic hormone; IPSS: inferior petrosal sinus sampling; SC: serum cortisol; UFC: 24-h urinary free cortisol; C:P: central:peripheral.
Normal values: SC (5.0–25.0 µg/dl), ACTH (5.0–60.0 pg/ml), UFC (20.0–120.0 μg/24 h).
Table 2 shows the association between the final diagnosis of ACTH-dependent CS (pituitary or ectopic) and the criteria for variation of SC and ACTH after CRH stimulation, as well as the criteria for C:P ACTH gradients (p < 0.01). The determination of the C:P ACTH gradient after CRH stimulation increased the sensitivity of IPSS, increasing the number of patients with detected CD from 17 to 19, without changes in specificity.
Sensitivity and specificity of the CRH and IPSS tests in patients with ACTH-dependent Cushing's Syndrome. Hospital General Universitario Gregorio Marañón 2004–2019.
Regarding the location of the adenoma in patients with CD, a total of nine out of 19 patients (47%) had a positive PMRI, with an average size of 4.50 (2.50–8.00) mm, with a right-sided location in five cases and left in four. The IPSS correctly predicted the location in seven patients (77%), in one patient it did not lateralise and in the remaining case the lateralisation located the adenoma on the opposite side to that observed in the PMRI. Regarding the analysis of patients with negative PMRI, the IPSS lateralised in all cases, and correct lateralisation was confirmed postoperatively in seven of the 10 patients (the rest were inoperable or had not been operated on at the date of this publication). In those patients with ECS, PMRI revealed a non-functional pituitary lesion in one patient and the IPSS did not lateralise in any case (interpetrosal gradient at baseline and after stimulation <1.4).
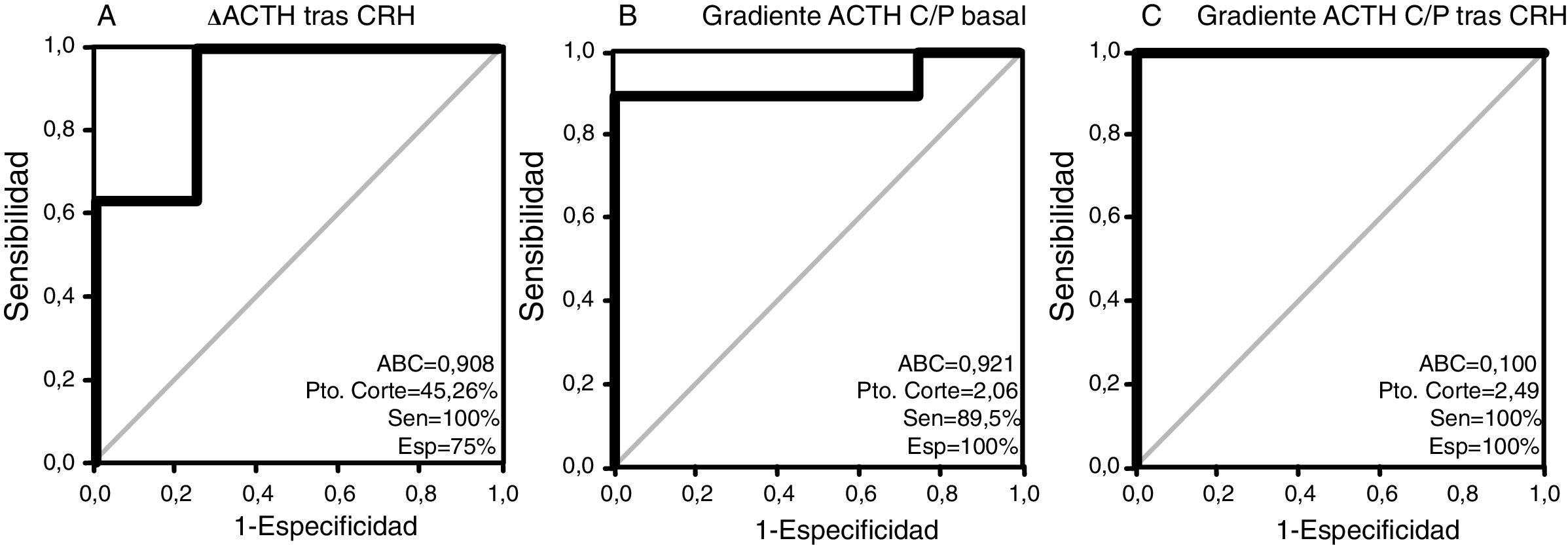
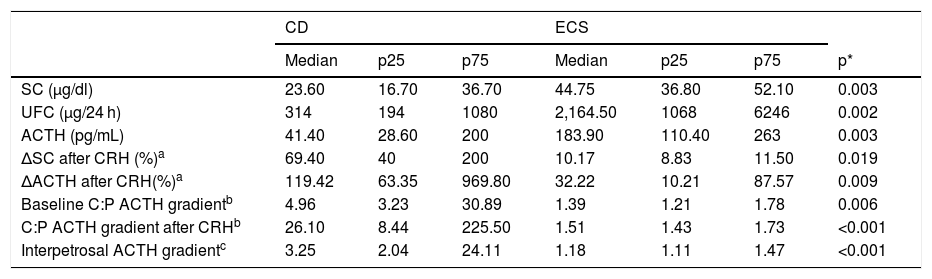
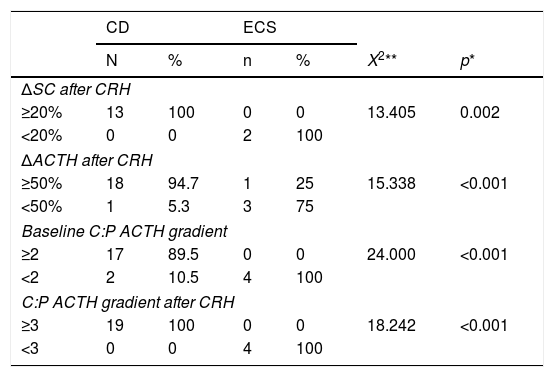
The results of the ROC analysis for all patients are shown in Fig. 1. The area under the curve (AUC) was 0.908 (95% CI, 0.736–1.068) for the CRH test and 0.921 (0.804–1.038) and 1000 (1000–1000) for the C:P ACTH gradient before and after stimulation, respectively. The optimal cut-off value for the CRH test (increase in ACTH after stimulation as a percentage) was 45.26%, with a sensitivity of 100% and a specificity of 75%. Regarding the C:P ACTH gradient in IPSS, the optimal cut-off value determined in our study was 2.06 for baseline (with a sensitivity of 89.5% and a specificity of 100%) and 2.49 after CRH stimulation (with a sensitivity and specificity of 100%).
DiscussionEstablishing a CS diagnosis is straightforward when the clinical signs and symptoms are florid and the functional and imaging tests are concordant, enabling the location of the disease to be correctly identified. However, some presentations of ACTH-dependent CS can represent a diagnostic challenge. This is particularly true in cases where imaging tests do not reveal significant findings, and where functional tests give inconsistent results.11 Differentiating between CS of pituitary and ectopic origin is a key issue in the treatment of ACTH-dependent CS, as they will require different therapeutic approaches. Surgical excision of the tumour by transsphenoidal surgery is the therapeutic option of choice in most patients with Cushing's disease, which is why identifying the correct location of the lesion is essential in order to correctly plan the surgery.
In our study, 19 patients had CD, while four were identified as ECS. This is consistent with the general trends reported in previous publications, where the prevalence of CD is significantly higher, ranging between 60–80% of ACTH-dependent CSs.12,13 Similarly, the average age of manifestation and the gender distribution in our study did not differ from that presented in the literature.14–17
The analysis of the biochemical parameters between the different groups also revealed some differential characteristics. Serum cortisol, 24-h urinary free cortisol and ACTH values were markedly higher in patients with ECS, consistent with the findings of previous studies.3,18,19 A nine-case series published by Araujo Castro et al.19 in Madrid is noteworthy. The authors found a mean urinary free cortisol of 2840 µg/dl in patients with ECS, which is close to the value obtained in our sample. However, the biochemical differences between the two groups are often not significant, probably due to the small number of patients with ectopic ACTH secretion in all series.
Therefore, performing functional tests that allow greater diagnostic sensitivity and specificity is of great assistance. The peripheral blood CRH stimulation test is a technique that can identify cases of pituitary CS by detecting percentage increases in serum ACTH levels above a certain value; classically an increase of fifty percent or more has been established as a cut-off point. In our investigation, an optimal cut-off point of 45.26%, very close to the traditional cut-off point, was identified, with a sensitivity of 94.7% and specificity of 75%. Ritzel et al.20 published similar results in a sample of 96 patients with CS. Similarly, in an Italian multicentre study by Giraldi et al.21 conducted in 160 patients with CS, the response to the CRH test was lower in subjects with adrenal lesions, and no significant increases in cortisol or ACTH were observed in this test in patients with ectopic ACTH secretion.
Imaging tests are useful for locating tumours. However, the results can be insufficient for two main reasons: up to 20–30% of secretory adenomas may not be visible on PMRI (false negative) or the findings may be inconclusive,22–26 and up to 10% may show a non-functional lesion or incidentaloma (false positive) that is not responsible for excess ACTH secretion.23–27 In their series of patients with ACTH-dependent CS, Yogi-Morren et al.4 found 23% of patients diagnosed with CS of ectopic origin to have pituitary incidentalomas, and just 68.3% of those diagnosed with CS of pituitary origin to have pituitary lesions. Other published series show similar results, revealing up to 20% of pituitary incidentalomas in ECS28 and PMRI detecting around 50% of corticotropic adenomas.5,29–31 In our series, one case of ECS presented with a pituitary incidentaloma, representing 25% of the total, and only 47% of the patients with CD had a PMRI that identified the lesion.
The introduction of diagnostic procedures such as IPSS constitute a valuable tool in this scenario.9 IPSS offers high sensitivity and specificity to distinguish between CS of central origin and of ectopic origin, and can be useful in locating tumours of pituitary lesions, with a good safety profile and tolerance for the patient.23
Traditionally, cut-off points have been established as two (baseline) and three (post-stimulation) to document a pituitary source of ACTH using IPSS. In the study by Oldfield et al. the reason why these cut-off values were chosen was not specified, but it can be inferred that they allowed for maximising sensitivity while maintaining 100% specificity.32 These cut-off points yielded excellent accuracy in this study. However, the optimal cut-off value may vary between different centres and studies33–35, and multicentre studies are required to clarify the heterogeneity of results36.
Our analysis with ROC curves established an optimal cut-off point for the baseline C:P ACTH gradient of 2.06, very similar to the traditional cut-off point,32 with a sensitivity of 89.5% and specificity of 100%, but the value after CRH stimulation had a lower cut-off point of 2.49 with a sensitivity and specificity of 100%. Similar results had already been observed in previous studies in which a lower cut-off point than is traditionally used was required to increase the sensitivity of the procedure.34–37 For example, in the retrospective study by Machado et al.33 that included 56 patients with ACTH-dependent CS, the cut-off point for the baseline C:P ACTH gradient was 1.45, with a sensitivity of 88.2% and a specificity of 100%. Similarly, in a study by Ilias et al.34 with 13 cases of ECS, the cut-off point for the baseline gradient was lowered to 1.6 to increase the sensitivity from 89% to 91%. Finally, the retrospective study carried out by Chen et al.36 was striking in that their analysis of the ROC curves established as optimal cut-off values 1.4 for the baseline C:P ACTH gradient and 2.8 after stimulation. In this same study, a meta-analysis that included 25 studies with a total of 1249 patients with CD and 152 with ECS who underwent IPSS was conducted. The aetiological diagnosis was determined with the traditional cut-off points, obtaining as results a sensitivity of 86% and 97% and a specificity of 98% and 100% before and after stimulation, respectively.
Despite being an excellent diagnostic test, the IPSS is not without its limitations. Depending on the series consulted, the percentage of false positives and false negatives may vary. Doppman et al.38 report a false negative rate of 0.8% in their sample of 501 patients, which were found to be due to the existence of a hypoplastic or anomalous petrosal sinus. False negatives may be due to poor catheter placement or asymmetric or anomalous venous drainage. Measurement of prolactin levels can help confirm correct placement.39 In contrast, the occurrence of false positives could result from incomplete suppression of the pituitary axis in cases of adrenal or ectopic CS, or in patients with cyclical or mild CS.40
Regarding the location of the pituitary adenoma in CD cases, our series showed a sensitivity in predicting laterality of 70% with the IPSS. Our results appear to be in line with previous studies.8,32 According to the latest guidelines, the role of IPSS in locating the microadenoma on the right or left side of the pituitary gland is much debated.8 In the study by Oldfield et al32, a baseline C:P ACTH gradient ≥1.4 predicted the location of the microadenoma with an accuracy of approximately 70%. However, it is worth noting a more recent study by Andereggen et al.41, which found the accuracy of IPSS to be much higher, correctly predicting the location of the adenoma in 96% of patients with CD. In contrast, in a study by Tabarin et al.42, IPSS identified an incorrect location in 41% of their sample, echoing several previous studies in which a failed location has been reported.43–47 Further multicentre studies are needed to shed light on this aspect.
This study has some important limitations, although they do not differ from those found in most previous studies.7,36,37 Given that CS is a rare condition, and especially cases of ECS, our sample of patients is relatively small, a fact that makes it difficult to extrapolate or generalise the results. The study of larger samples could yield ROC curves with smoother slopes and a better reflection of the diagnostic power of these procedures and methods in practice. Finally, we found it difficult to postoperatively confirm CD in some patients given that they continued with follow-up in their hospitals of origin for surgery and these data could not be completed, or they did not undergo surgery.
Finally, we experienced a certain amount of difficulty collecting data in older patients or those from other hospitals for whom medical reports were incomplete and we did not have a full description of the pathological anatomy. Moreover, due to the age of the sample, prolactin levels were not measured in this study.
ConclusionsIPSS is a technique with high sensitivity and specificity in the diagnostic procedure of ACTH-dependent CS, and is very useful, especially in dubious cases, without involving high risks. The optimal cut-off value of the C:P ACTH gradient for the diagnosis of CD may vary between the different studies and from the traditionally established cut-off value, so we recommend that each experienced centre evaluate their own cut-off points.
The CRH stimulation test and IPSS are very useful techniques in the diagnostic procedure of ACTH-dependent CS for being able to correctly classify patients with CD and ECS. Our study found an optimal cut-off point for the increase in ACTH after stimulation of 45.26%, which is close to the traditional value. The optimal cut-off value for IPSS was 2.06 before stimulation, but 2.49 after stimulation, which differs from the point used with the classical criteria. In this case, it is demonstrated that a C:P ACTH gradient of less than three after stimulation does not exclude the central origin of ACTH-dependent CS; however, no differences were found in the gradient without stimulation. We therefore recommend that each experienced centre evaluate its own cut-off points.
FundingThis study has not received any type of funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: González Fernández L, Añez Ramos RJ, Rivas Montenegro AM, Brox Torrecilla N, Miguélez González M, Muñoz Moreno D, et al. Cateterismo de senos petrosos inferiores y estimulación con CRH: 15 años de experiencia en un hospital de tercer nivel. Endocrinol Diabetes Nutr. 2021;68:381–388.