The impact of subclinical hypothyroidism (SH) and thyroid autoimmunity on obstetric and perinatal complications continues to be a matter of interest and highly controversial.
AimTo assess the impact of SH and autoimmunity in early pregnancy on the obstetric and perinatal complications in our population.
Material and methodA retrospective cohort study in 435 women with SH (TSH ranging from 3.86 and 10μIU/mL and normal FT4 values) in the first trimester of pregnancy. Epidemiological and clinical parameters were analyzed and were related to obstetric and perinatal complications based on the presence of autoimmunity (thyroid peroxidase antibodies [TPO]>34IU/mL).
ResultsMean age was 31.3 years (SD 5.2). Seventeen percent of patients had positive TPO antibodies. Presence of positive autoimmunity was associated to a family history of hypothyroidism (p=0.04) and a higher chance of miscarriage (p=0.009). In the multivariate analysis, positive TPO antibodies were associated to a 10.25-fold higher risk of miscarriage. No statistically significant associations were found with all other obstetric and perinatal complications.
ConclusionsIn our region, pregnant women with SH and thyroid autoimmunity had a higher risk of miscarriage but not of other obstetric and perinatal complications.
El impacto del hipotiroidismo subclínico (HSC) y la autoinmunidad antitiroidea positiva en los resultados obstétricos y perinatales permanece en controversia y es objeto de gran interés.
ObjetivoEvaluar el impacto del HSC y la autoinmunidad positiva en las complicaciones obstétricas y perinatales en nuestra población.
Material y métodoEstudio de cohortes retrospectivo en 435 mujeres con HSC (TSH entre 3,86 y 10μUI/ml, y FT4 normal) en el primer trimestre de la gestación, con seguimiento durante el embarazo. Se analizaron parámetros epidemiológicos y clínicos y se relacionaron con complicaciones obstétricas y perinatales en función de la presencia de autoinmunidad positiva (anticuerpos antiperoxidasa [aTPO] > 34 UI/ml).
ResultadosLa edad media fue de 31,3 años (desviación estándar: 5,2). El 17% de las pacientes presentaban aTPO positivos. La presencia de aTPO se asoció a antecedentes familiares de hipotiroidismo (p = 0,04), y con una mayor probabilidad de aborto (p = 0,009). En el análisis multivariante, los aTPO positivos suponían un aumento de probabilidad de presentar aborto de 10,25 veces. No se encontraron asociaciones estadísticamente significativas con el resto de las complicaciones obstétricas y perinatales.
ConclusionesEn nuestro medio, las gestantes con HSC y autoinmunidad positiva presentan un mayor riesgo de aborto, pero no de otras complicaciones obstétricas y perinatales.
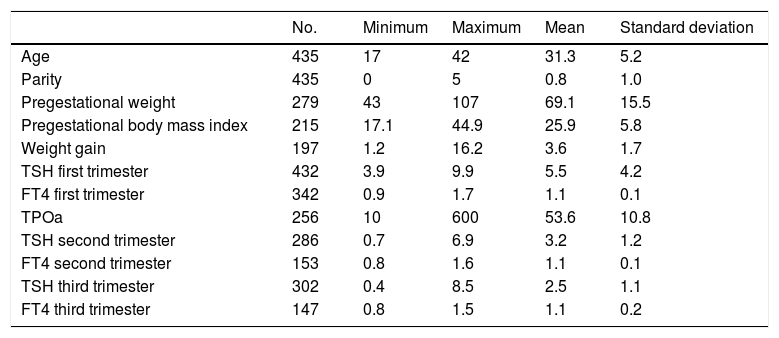
Pregnancy is characterized by endocrine changes that give rise to thyroid hormone (TH) values different from those of the general population (Table 1).1 In this regard, TH reference values (RVs) stratified by trimesters and in each reference population are needed.2
Descriptive results referring to the epidemiological, clinical and metabolic parameters.
| No. | Minimum | Maximum | Mean | Standard deviation | |
|---|---|---|---|---|---|
| Age | 435 | 17 | 42 | 31.3 | 5.2 |
| Parity | 435 | 0 | 5 | 0.8 | 1.0 |
| Pregestational weight | 279 | 43 | 107 | 69.1 | 15.5 |
| Pregestational body mass index | 215 | 17.1 | 44.9 | 25.9 | 5.8 |
| Weight gain | 197 | 1.2 | 16.2 | 3.6 | 1.7 |
| TSH first trimester | 432 | 3.9 | 9.9 | 5.5 | 4.2 |
| FT4 first trimester | 342 | 0.9 | 1.7 | 1.1 | 0.1 |
| TPOa | 256 | 10 | 600 | 53.6 | 10.8 |
| TSH second trimester | 286 | 0.7 | 6.9 | 3.2 | 1.2 |
| FT4 second trimester | 153 | 0.8 | 1.6 | 1.1 | 0.1 |
| TSH third trimester | 302 | 0.4 | 8.5 | 2.5 | 1.1 |
| FT4 third trimester | 147 | 0.8 | 1.5 | 1.1 | 0.2 |
TPOa: peroxidase antibodies.
In the absence of local values, both the Spanish Society of Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición [SEEN]) and the American Thyroid Association (ATA)3 recommend the following RVs: 0.1–2.5μIU/ml in the first trimester; 0.2–3.0μIU/ml in the second trimester; and 0.3–3.0μIU/ml in the last trimester of pregnancy. The value of 2.5μIU/ml was selected not only because it is close to percentile (P) 97.5, but also because higher values are associated with greater fetal morbidity. However, in other regions higher TSH percentile 97.5 results are reported.1,4,5 Accordingly, the reference intervals for both TSH and FT4 in pregnant women proposed by the ATA could lead to an overdiagnosis of subclinical hypothyroidism (SCH). In fact, it is particularly significant that the reference intervals for TSH in the Spanish and Andalusian populations corresponding to very different geographical areas, and obtained using different laboratory techniques and statistical methods, differ markedly from the ATA cut-off point, and are very similar to each other.6
It is well known that clinical hypothyroidism (CH) is related to maternal and fetal adverse effects.7 In the case of SCH the evidence is weaker, however. Although some studies have described an association between SCH and premature delivery, miscarriage, gestational hypertension, gestational diabetes, fetal distress, placental anomalies and low birth weight,8 other studies have shown no evidence of this.9 An increased risk of miscarriage, premature delivery and retarded intellectual and motor development has been associated with thyroid peroxidase antibody (TPOa) positivity.10,11
The effects of both SCH and thyroid gland autoimmunity upon the obstetric and perinatal outcomes remain subject to controversy and are of great interest. First of all, their evaluation requires the definition of a true RV in the study population. Accordingly, in our setting we established the median and confidence interval (CI) in a total of 1082 women in the first trimester of pregnancy, obtaining a TSH cut-off point (percentile 97.5) of 3.86μIU/ml (95%CI: 3.71–3.99).
The aim of this study was to evaluate the impact of SCH (defined on the basis of our RVs) with positive autoimmune findings upon the obstetric and perinatal outcomes in our healthcare area.
Material and methodsA prospective observational study was carried out in 435 patients with SCH defined on the basis of our previously established local RVs (TSH between 3.86 and 10μIU/ml, with normal FT4) in the first trimester of pregnancy (TPOa>34IU/ml), and subjected to follow-up during pregnancy and up to delivery in a joint Endocrinology and Obstetrics clinic of Hospital Universitario Puerta del Mar (Cádiz, Spain). The case histories of the women attending the clinic from January 2015 until the end of pregnancy in January 2017 were compiled. The patients with SCH amenable to inclusion in the study were recruited by non-probabilistic sampling, in a consecutive sample of cases.
The following inclusion criteria were applied: women in the first trimester of pregnancy (weeks 10–12), with normal gestation (presenting no exclusion criteria) and TSH levels between 3.85 and 10μIU/ml, with normal FT4 (0.93–1.7ng/dl). The following exclusion criteria were applied: women in the first trimester of pregnancy (weeks 10–12), with TSH concentrations between 3.85 and 10μIU/ml and low FT4, a diagnosis of placental insufficiency, pregestational diabetes, chronic underlying systemic disease or acute infection, known previous thyroid disorders, and multiple pregnancy.
The study variables were collected from the case history, obstetric history and clinical interview of the patient. Data collection for the study was divided into two phases. The first phase involved the compilation of data regarding the identification of the patient (age, obstetric history, number of pregnancies or parity, weight before pregnancy and pregestational body mass index) and laboratory test results. In those cases where TSH was >3.85μIU/ml, the determination of FT4 and TPOa was carried out automatically. The second phase involved consultation of the case histories to determine the evolution of TSH and FT4 concentrations in the second trimester (24–26 weeks of gestation) and third trimester (32–34 weeks of gestation). Furthermore, we documented the complications of pregnancy (intrauterine growth retardation [IUGR], preeclampsia, arterial hypertension [AHT] induced by pregnancy [AHT diagnosed after week 20], chronic AHT [both manifesting after week 20 and pregestational AHT], chronic AHT with added preeclampsia, eclampsia, gestational diabetes, threatened preterm delivery, preterm delivery, risk of loss of fetal wellbeing, miscarriage, abruptio placentae and intrauterine fetal death), gestational age at birth, type of delivery, and newborn infant weight (low weight or macrosomia) and the Apgar score.
The study was approved by the Research Ethics Committee of Hospital Universitario Puerta del Mar. The international ethical recommendations of the Declaration of Helsinki were followed. The study complied with the specifications of Act 14/2007, of 3 July, referring to Biomedical Research, and of Act 14/2002, of 14 November, regulating patient autonomy and rights and obligations regarding clinical documentation and information. Data anonymity was observed during the analysis of the case histories.
Statistical analysisThe Kolmogorov–Smirnov test was used to assess data goodness of fit to a normal distribution. Frequencies and percentages were calculated for the qualitative variables, while quantitative variables were reported as the mean, standard deviation (SD) and minimum and maximum values. The student t-test was used for the comparison of means between two categories of an independent qualitative variable (SCH and positive autoimmunity) and a quantitative variable such as the week of termination of pregnancy and newborn infant weight with respect to the presence of SCH, positive autoimmunity and the Apgar score. The chi-squared test was used to compare a qualitative variable with another independent nominal qualitative variable, both corresponding to two groups, such as the type of delivery and the other maternal-fetal complications (IUGR, preeclampsia, AHT induced by pregnancy, chronic AHT, eclampsia, gestational diabetes, threatened preterm delivery, preterm delivery, risk of loss of fetal wellbeing, miscarriage, and intrauterine fetal death), in relation to SCH and positive autoimmunity. The magnitude of the associations was calculated from the relative risk (RR), with assessment of the precision of the estimates based on the 95%CI, using the Cornfield approach. Lastly, multivariate logistic regression analysis was performed to check the association of simultaneous qualitative variables. The stepwise technique was used to select independent variables for inclusion in the model. Statistical significance was considered for p<0.05 in all cases.
The data were processed and analyzed using the SPSS version 20.0 statistical package for MS Windows, with the fields corresponding to the variables measured in the study.
ResultsWe compiled a total of 435 case histories of pregnant women with SCH. Of these, 74 presented positive autoimmunity (17%). Table 1 shows the results of the descriptive quantitative variables referring to the epidemiological, clinical and laboratory test parameters. A total of 328 pregnant women (75.4%) had no family history of thyroid gland disease. Ten percent of the pregnancies were the result of in vitro fertilization (IVF) techniques.
With regard to the obstetric and fetal complications, 39 patients presented gestational diabetes (9%), one patient suffered from preeclampsia, 8 presented AHT induced by pregnancy, two patients had chronic AHT (0.5%) and 10 suffered threatened preterm delivery (2.3%). In turn, 12 preterm deliveries were recorded (2.8%), as well as 6 miscarriages (1.4%) after an average of 12.6 weeks (SD 3.1) of gestation, and 6 intrauterine fetal deaths (1.4%). There were no cases of abruptio placentae. On the other hand, assisted vaginal delivery occurred in 51% of the cases, with cesarean section in 16.1%. The estimated mean gestational age at birth was 39.4 weeks (SD 1.2). The mean newborn infant weight was 3292.6g (SD 462.4). The mean Apgar score was 9.92 (SD 0.33). Five fetuses presented IUGR (1.1%), and 7 cases of risk of loss of fetal wellbeing were recorded (1.7%), as well as 16 infants with low birth weight (3.7%) and 25 cases of macrosomia (5.7%).
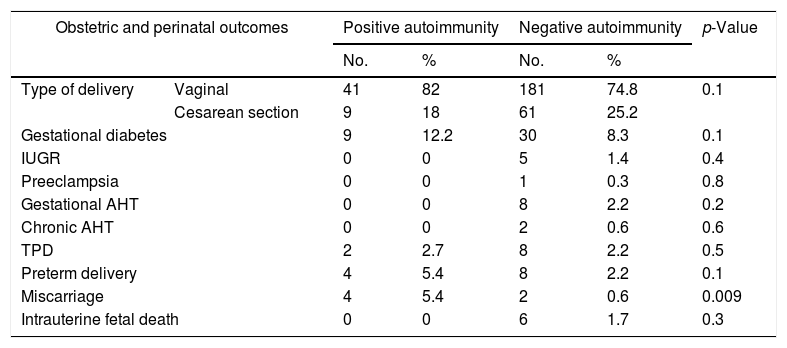
The results referring to obstetric-perinatal complications in relation to the presence of autoimmunity are shown in Table 2. Positive autoimmunity was seen to be associated with an increased risk of miscarriage (p=0.009). No statistically significant differences were found with regard to the rest of the maternal and fetal outcomes. The same applied to gestational age at birth, newborn infant weight and the Apgar score after 5min.
Comparison of obstetric and perinatal outcomes according to the presence of autoimmunity.
| Obstetric and perinatal outcomes | Positive autoimmunity | Negative autoimmunity | p-Value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Type of delivery | Vaginal | 41 | 82 | 181 | 74.8 | 0.1 |
| Cesarean section | 9 | 18 | 61 | 25.2 | ||
| Gestational diabetes | 9 | 12.2 | 30 | 8.3 | 0.1 | |
| IUGR | 0 | 0 | 5 | 1.4 | 0.4 | |
| Preeclampsia | 0 | 0 | 1 | 0.3 | 0.8 | |
| Gestational AHT | 0 | 0 | 8 | 2.2 | 0.2 | |
| Chronic AHT | 0 | 0 | 2 | 0.6 | 0.6 | |
| TPD | 2 | 2.7 | 8 | 2.2 | 0.5 | |
| Preterm delivery | 4 | 5.4 | 8 | 2.2 | 0.1 | |
| Miscarriage | 4 | 5.4 | 2 | 0.6 | 0.009 | |
| Intrauterine fetal death | 0 | 0 | 6 | 1.7 | 0.3 | |
TPD: threatened preterm delivery; IUGR: intrauterine growth retardation; AHT: arterial hypertension.
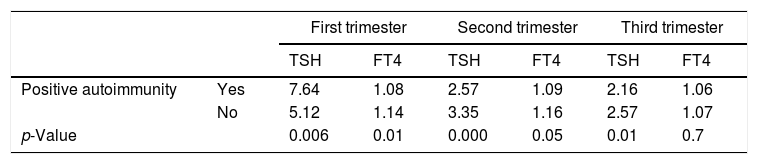
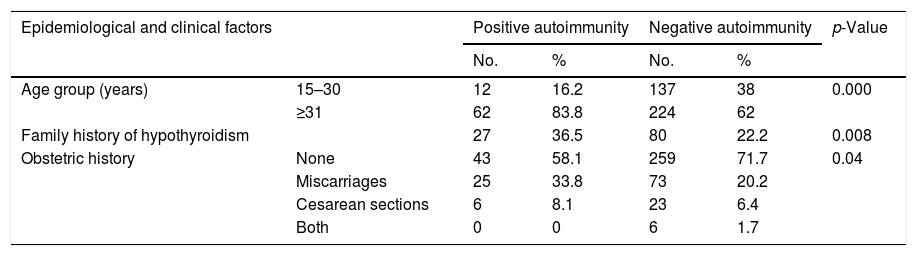
Table 3 shows the TSH levels to be higher in the different trimesters among the patients with positive autoimmunity. In our study series, the presence of positive autoimmunity was associated with a patient age of over 31 years, a family history of hypothyroidism, and a history of adverse obstetric events (miscarriages or cesarean section). There were no significant differences in the number of previous pregnancies, maternal body weight or weight gain between the pregnant women with autoimmunity and those without (Table 4).
Mean TSH and thyroid hormone values in the pregnant women according to the presence of positive autoimmunity.
| First trimester | Second trimester | Third trimester | |||||
|---|---|---|---|---|---|---|---|
| TSH | FT4 | TSH | FT4 | TSH | FT4 | ||
| Positive autoimmunity | Yes | 7.64 | 1.08 | 2.57 | 1.09 | 2.16 | 1.06 |
| No | 5.12 | 1.14 | 3.35 | 1.16 | 2.57 | 1.07 | |
| p-Value | 0.006 | 0.01 | 0.000 | 0.05 | 0.01 | 0.7 | |
TSH: IU/ml; FT4: ng/dl.
Epidemiological, clinical and laboratory test parameters in relation to autoimmunity.
| Epidemiological and clinical factors | Positive autoimmunity | Negative autoimmunity | p-Value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Age group (years) | 15–30 | 12 | 16.2 | 137 | 38 | 0.000 |
| ≥31 | 62 | 83.8 | 224 | 62 | ||
| Family history of hypothyroidism | 27 | 36.5 | 80 | 22.2 | 0.008 | |
| Obstetric history | None | 43 | 58.1 | 259 | 71.7 | 0.04 |
| Miscarriages | 25 | 33.8 | 73 | 20.2 | ||
| Cesarean sections | 6 | 8.1 | 23 | 6.4 | ||
| Both | 0 | 0 | 6 | 1.7 | ||
The logistic regression analysis showed positive autoimmunity to be related to a 10.25-fold increase in the probability of miscarriage versus the patients with negative autoimmunity (exponent [B]=10.25).
DiscussionThe aim of this study was to investigate the association between TPOa positivity in pregnant women with SCH and the appearance of obstetric and perinatal complications. Positive autoimmunity was recorded in 17% of the patients, a figure that is consistent with the findings of other studies.12 Santiago et al.12 recorded a progressive increase in TSH concentrations in the course of pregnancy, with a parallel decrease in FT4 values. In our series, the mean TSH concentration was seen to decrease over each successive trimester, with values of 5.55μIU/ml, 3.21μIU/ml and 2.49μIU/ml, respectively, while FT4 levels decreased only in the last trimester. On the other hand, TSH values were higher in the TPOa-positive women, matching the observations of Bocos-Terraz et al.,13 who found pregnant women with positive autoimmunity to have higher mean TSH levels than pregnant women without autoimmunity–except in the last trimester of pregnancy–while their FT4 levels were significantly lower in all but the last trimester of pregnancy.12 However, although in this study a direct relationship was observed between age and positive autoimmunity, other authors have concluded that maternal age is only associated with TSH elevation in the absence of antibodies.13
Our data only indicated a significant relationship between miscarriage and SCH associated with positive thyroid autoimmunity. The association between the risk of miscarriage and the presence of positive autoimmunity has been reported by different authors.7,14,15 In our study, positive autoimmunity was seen to be related to a 10.25-fold increase in the probability of miscarriage versus the patients with negative autoimmunity. Nevertheless, this observation must be interpreted with caution, since the risk of miscarriage is associated with a range of variables, including maternal age, a personal and family history of repeated miscarriage, and medical comorbidities,3 as we have also seen. With regard to the effect of levothyroxine treatment, the study published by Negro et al.16 consisted of a group of 57 pregnant women with TPOa positivity treated with levothyroxine, another group of 58 patients with TPOa positivity not treated with the drug, and a control group of 869 patients with negative autoimmunity. The miscarriage rates were significantly higher in the untreated TPOa-positive women than in the other two groups.
A number of authors have found no relationship between thyroid gland dysfunction and TPOa positivity with respect to newborn infant weight, the cesarean section rate, newborn infant admission to intensive care, or the development of gestational diabetes or preeclampsia in the mother. However, an increase in preterm deliveries has been reported when TPOa positivity is associated with SCH.17 A recent meta-analysis has found SCH to be associated with an increased frequency of IUGR. This was not observed when SCH was associated with positive autoimmunity, however.18 We likewise observed no such relationship. This is probably because women presenting SCH with or without positive autoimmunity who are asymptomatic and suffer mild thyroid gland dysfunction are not actually at greater risk of suffering other obstetric complications. In fact, a recent study carried out by Plowden et al.19 concluded that SCH and thyroid autoimmunity are not associated with an increased risk of premature delivery, gestational diabetes or preeclampsia. Their data therefore support recent recommendations to the effect that low risk asymptomatic women should not be subjected to systematic testing for the detection of thyroid gland dysfunction or autoimmunity.
Due to the firm association between CH and obstetric and perinatal outcomes, women with CH should always be treated. However, levothyroxine treatment in SCH is not universally recommended, due to the scant evidence of the benefits of such therapy. A randomized study showed that levothyroxine treatment in pregnant women with SCH reduces the incidence of adverse events in both the mother and the fetus.17 An analysis of a subgroup of the CATS study found thyroid gland dysfunction to be associated with adverse obstetric outcomes, while levothyroxine treatment was seen to be able to improve these outcomes.20 However, in another recent randomized, double-blind, placebo-controlled trial, levothyroxine treatment in patients with SCH did not improve the cognitive outcomes among their offspring at 5 years of age.21
At present, levothyroxine treatment is advised in pregnant women with SCH associated with TPOa positivity, and such treatment is, moreover, considered to be of potential benefit even in the absence of positive autoimmunity.22,23 In fact, according to a recent study,24 levothyroxine treatment is associated with a lesser risk of miscarriage in women with SCH, particularly those presenting pre-treatment TSH concentrations of 4.1–10mIU/l. Thus, on the basis of our results, and while awaiting new supporting interventional studies, all pregnant women with SCH, particularly in the presence of associated positive autoimmunity, should be treated with levothyroxine from the first trimester of pregnancy.
ConclusionsPregnant women with SCH, defined according to our local RVs, associated with positive autoimmunity, are at an increased risk of miscarriage and should receive levothyroxine treatment. The presence of positive thyroid autoimmunity is associated with older maternal age, higher TSH and FT4 levels, and a family history of thyroid gland disease.
AuthorshipCLT and MAF designed the study. ARM and ALB conducted the fieldwork and entered the data for analysis. ASB was in charge of obtaining our thyroid hormone RVs. CLT, MAF, ARM and ALB prepared the first manuscript draft and incorporated the suggestions of all the co-authors. All the authors read and approved the final manuscript version.
Conflicts of interestThe authors state that they have no conflicts of interest capable of jeopardizing the impartiality of this scientific study.
Please cite this article as: López-Tinoco C, Rodríguez-Mengual A, Lara-Barea A, Barcala J, Larrán L, Saez-Benito A, et al. Impacto de la autoinmunidad antitiroidea positiva en gestantes con hipotiroidismo subclínico. Endocrinol Diabetes Nutr. 2018;65:150–155.