Hyponatraemia, defined as a blood sodium level below 135 mEq/l, is the most common electrolyte abnormality in patients with heart failure (HF). It is present in 13%–15% of cases, whether patients have a decreased or preserved ejection fraction and whether they are seen on an outpatient basis1 or hospitalised.2 This abnormality is considered a marker of a poor prognosis, as a significant inverse relationship is seen between blood sodium level and mortality, with more re-hospitalisations, longer hospital stays and higher rates of morbidity and mortality over a long-term period.3–5 Patients with hyponatraemia also show a worse response to treatment, requiring higher doses of diuretics to achieve a level of diuresis similar to patients with normal blood sodium levels, especially in those with sodium levels below 130 mEq/dl.6
The current options for treating hyponatraemia in HF include: hypertonic saline solution, fluid restriction and drugs such as loop diuretics, lithium carbonate, demeclocycline and urea. Vasopressin receptor antagonists such as tolvaptan, lixivaptan and conivaptan show promise in this indication. Fluid restriction causes a minimal increase in sodium, with persistently high hyponatraemia.7 Demeclocycline (a tetracycline) and lithium carbonate can have serious cardiovascular and renal side effects. Urea in hyponatraemia has had an indication in ascites, cirrhosis and syndrome of inappropriate secretion of antidiuretic hormone (SIADH), and may be a more affordable alternative to tolvaptan in HF.
Urea is an organic compound of carbon, nitrogen, oxygen and hydrogen that is produced in the liver. An osmotic diuretic, it increases plasma osmolarity, draws water from the extracellular compartment and causes an increase in urine osmolarity which promotes water excretion and yields a reduction in natriuresis. Certain brief publications, some of them older, on the use of oral urea (there is an intravenous formulation) in HF with hyponatraemia have demonstrated safety and effectiveness, in particular in SIADH; however, it is not widely indicated in HF.8,9
This retrospective observational study evaluated normalisation of sodium levels (Na+ = 135 ± 3 mEq/l) with treatment using oral urea in patients with HF and hyponatraemia (Na+ <135 mEq/l), whether hospitalised or not, being followed up by the heart failure unit between January 2013 and May 2018. Data collection was done by reviewing electronic medical records. The exclusion criteria were: blood glucose >250 or 180−250 mg/dl plus Na+ 133−135 mEq/l on admission, serious kidney failure (glomerular filtration rate <30 ml/min/1.73 m2) and serious liver disease.
A total of 34 patients, all on standard treatment for HF, were included. Their mean age was 79.94 years; 24 (70.58%) were women; 25 (73.5%) were hospitalised; nine (26.5%) were seen on an outpatient basis; 29 (85.3%) had hypertension; 16 (47.1%) had diabetes; 18 (52.9%) had dyslipidaemia; and 20 (58.8%) had atrial fibrillation. Regarding aetiology, 16 (47.1%) had hypertensive heart failure, 11 had (32.4%) ischaemic heart failure and seven (20.6%) had dilated heart failure. Ejection fraction was preserved (>50%) in 19 (55.9%) patients, intermediate (40%–50%) in five (14.7%) patients and depressed (<40%) in 10 (29.4%) patients. The New York Heart Association (NYHA) Functional Classification was I in 0 (0%) patients, II in 11 (32.4%) patients, III in 19 (55.9%) patients and IV in four (11.8%) patients. Glomerular filtration rate, estimated using the MDRD equation (ml/min/1.73 m2), was 30–60 in 58.8% and >60 in 47.1%. N-terminal pro–B-type natriuretic peptide (BNP) (pg/mL) was 7,283.69 ± 8,752.30. Drug treatment consisted of oral furosemide in nine (26.5%) patients; intravenous furosemide in 25 (73.5%) patients; thiazides in six (17.6%) patients; spironolactone in 19 (55.9%) patients; angiotensin-converting enzyme (ACE) inhibitors/angiotensin-II receptor blockers (ARBs) in 21 (61.1%) patients and beta blockers in 25 (73.5%) patients.
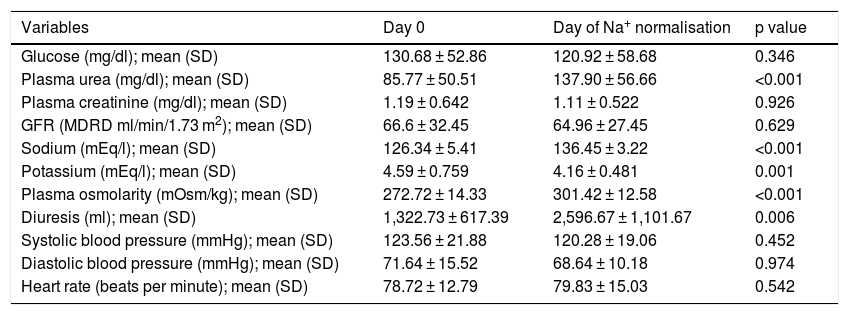
The mean dose of oral urea was 22.5 g/day. Blood sodium levels were 126.34 ± 5.41 mEq/l initially, 126.75 mEq/l after 24 h and 129.74 mEq/l after 48 h, and the mean on the day of normalisation was 136.45 ± 3.22 mEq/l (p < 0.001). Days to achieve normalisation of sodium levels were 4.28 ± 2.37 days. Uraemia at the start of treatment was 85.77 ± 50.51 mg/dl, and the mean on the day of Na+ normalisation was 137.90 ± 56.66 mg/dl (p < 0.001). When urea was added, there were clinically significant increases in diuresis volume compared to baseline (1,322.73 ml/day): 2,284.23 ml/day (p < 0.002) after 24 h, 2,586.45 ml/day (p < 0.005) after 48 h and 2,596.67 ml/day on the day of normalisation (an increase of 96.31%; p < 0.006). There were also significant changes in plasma osmolarity (p < 0.001) and a slight decrease in serum potassium (p < 0.001). There were no significant changes in creatinine levels or glomerular filtration rate estimated using the MDRD equation.
Changes over time in clinical and laboratory parameters from the effects of oral urea are summarised in Table 1.
Changes over time in laboratory and clinical parameters.
| Variables | Day 0 | Day of Na+ normalisation | p value |
|---|---|---|---|
| Glucose (mg/dl); mean (SD) | 130.68 ± 52.86 | 120.92 ± 58.68 | 0.346 |
| Plasma urea (mg/dl); mean (SD) | 85.77 ± 50.51 | 137.90 ± 56.66 | <0.001 |
| Plasma creatinine (mg/dl); mean (SD) | 1.19 ± 0.642 | 1.11 ± 0.522 | 0.926 |
| GFR (MDRD ml/min/1.73 m2); mean (SD) | 66.6 ± 32.45 | 64.96 ± 27.45 | 0.629 |
| Sodium (mEq/l); mean (SD) | 126.34 ± 5.41 | 136.45 ± 3.22 | <0.001 |
| Potassium (mEq/l); mean (SD) | 4.59 ± 0.759 | 4.16 ± 0.481 | 0.001 |
| Plasma osmolarity (mOsm/kg); mean (SD) | 272.72 ± 14.33 | 301.42 ± 12.58 | <0.001 |
| Diuresis (ml); mean (SD) | 1,322.73 ± 617.39 | 2,596.67 ± 1,101.67 | 0.006 |
| Systolic blood pressure (mmHg); mean (SD) | 123.56 ± 21.88 | 120.28 ± 19.06 | 0.452 |
| Diastolic blood pressure (mmHg); mean (SD) | 71.64 ± 15.52 | 68.64 ± 10.18 | 0.974 |
| Heart rate (beats per minute); mean (SD) | 78.72 ± 12.79 | 79.83 ± 15.03 | 0.542 |
GFR: glomerular filtration rate; SD: standard deviation.
There was one mild case of uraemic encephalopathy in an individual with a glomerular filtration rate of 32 ml/min/1.73 m2 and previously elevated blood urea levels (>150 mg/dl), which resolved in 48 h following suspension of the drug. Another patient stopped treatment, citing the bad taste of the urea preparation. There were five cases (14.70%) of asymptomatic hypotension (systolic blood pressure <100 and/or diastolic blood pressure <60 mmHg) during treatment. With respect to mortality, 30 days after starting treatment there were four cases (11.43%) with no increase after 60 days. The causes of mortality were infection (one case) and refractory HF (three cases).
The limitations of our study were its lack of a control group and its small sample size. Randomised controlled trials are needed to confirm the benefits of oral urea in patients with HF and hyponatraemia.
In conclusion, this study demonstrates the safety and efficacy of oral urea to correct hyponatraemia in patients with advanced HF; used on a short-term basis, this treatment causes no kidney damage and yields increased diuresis, such that it may have an indication in relieving HF symptoms.
One drawback of urea is its poor palatability, which may lead patients not to take it again. It should be borne in mind that the use of urea is contraindicated in advanced kidney failure, intracranial bleeding and liver failure.
Please cite this article as: Martínez Á, Rodríguez A, Corral M, Reyes E, Rodríguez S. Tratamiento de la hiponatremia en la insuficiencia cardiaca con urea oral. Endocrinol Diabetes Nutr. 2022;69:303–304.