Fournier's gangrene (FG) is a rare condition first described in 1883 and characterized by type I necrotizing fasciitis, with rapid spread to the perineal, genital and perianal region leading to thrombosis of small subcutaneous vessels secondary to obliterating endarteritis and necrosis of the underlying adipose tissue.1 The main risk factors for the development of FG are type 2 diabetes mellitus (DM2), chronic alcoholism, malignant neoplasms and immune deficiencies.2 There are also predisposing factors such as trauma, perianal abscesses, folliculitis or urological manipulations.3
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are currently used for the treatment of T2DM and improve glycemic control by inhibiting the reabsorption of filtered glucose in the proximal tubules of the kidney, thus incrementing urinary glucose excretion. The most common complications of the use of SGLT2i are genital mycotic and urinary tract infections. Urosepsis, pyelonephritis, ketoacidosis and acute renal failure have also been identified as adverse events of SGLT2i since the approval of these drugs.4 It was recently postulated that patients with diabetes treated with SGLT2i appeared to have a higher incidence of FG, particularly necrotizing fasciitis of the perineum and genitals, which in turn led to the publication of an alert by the United Stated Food and Drug Administration (FDA) in August 2018.5
The present study describes the case of a patient treated with dapagliflozin who suffered FG, followed by a literature review.
The patient was a 68-year-old male with a 13-year history of DM2 treated with metformin/sitagliptin 1000/50mg every 12h (since January 2015), dapagliflozin 10mg daily (since February 2017) and insulin glargine 20IU daily (since March 2018). Despite such treatment, metabolic control proved regular (glycosylated hemoglobin [HbA1c] concentration in May 2019: 7.8 %). Renal function at the start of treatment with dapagliflozin was normal (glomerular filtration rate [GFR]>60ml/min). The patient history moreover comprised arterial hypertension, dyslipidemia, chronic ischemic heart disease, vertebrobasilar stroke in 2017, prostate cancer in 2002 subjected to radiotherapy and in complete remission, and right nephrectomy due to renal angiomyolipoma. The patient carried a suprapubic catheter due to urethral stenosis, with end-to-end urethroplasty in September 2019.
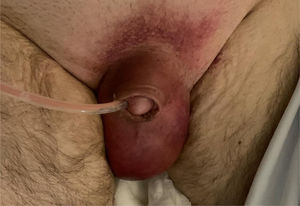
He reported to the emergency room 48h after discharge due to syncope, head injury and fever (38°C). Physical examination in the emergency room revealed blood pressure 142/84mmHg, heart rate 114bpm, body temperature 38°C, an erythematous scrotum with intense inflammation, and increased local temperature (Fig. 1). The blood tests showed hemoglobin 9.5g/dl, mean corpuscular volume 109fl, platelet count 251,000/mm3, leukocyte count 23,200/mm3, with 94% neutrophils, fibrinogen >1000mg/dl, INR 1.26, glucose 137mg/dl, C-reactive protein 29.8mg/dl and procalcitonin 2.07μg/l. The other parameters were normal. Abdominopelvic computed tomography revealed fluid bands and multiple gas bubbles in the perineal region extending to the scrotum, periurethral zone and pubis, these findings being consistent with Fournier's gangrene. Blood cultures were made and empirical antibiotic therapy was started with meropenem 1g/8h, daptomycin 10mg/kg/24h and clindamycin 600mg/8h. Urine culture proved positive for extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and carbapenemase-producing Pseudomonas aeruginosa. The blood cultures were negative. The perineal incision was opened, with cleaning and debridement, and an intraoperative culture sample was obtained. Regarding the surgical wound, the S. pyogenes rapid test was negative, and culture proved positive for ESBL-producing E. coli, P. aeruginosa, Morganella morganii and Enterococcus sp. The clinical course proved to be slow, requiring multiple surgical debridement procedures and antibiotic therapy. During his 9-week hospital stay, blood glucose control was regular, with frequent hyperglycemia peaks and glycemia in the range of 80–320mg/dl. The patient was discharged home in an acceptable clinical condition and carrying a suprapubic catheter. He was able to walk and was prescribed glucose-lowering treatment in the form of metformin/sitagliptin and insulin glargine.
Fournier's gangrene is an extremely rare condition affecting 1.6/100,000 males and covering a wide age range (18–87 years).1,6 The underlying physiopathological mechanisms are not fully clear, but there appears to be greater endothelial damage at the microvascular level. The condition is classified according to its anatomical origin into intestinal, urinary, testicular and cutaneous FG. The disease is associated with a high mortality and a long-term intensive care stay, conditioned by comorbidities, demographic factors, diagnostic delays, intervention time, and prognostic scores such as the Fournier Gangrene Severity Index.2,6 Fournier's gangrene is usually a polymicrobial infection, and the most commonly isolated organisms are: E. coli, Streptococcus, Bacteroides, Enterococcus, Pseudomona, Corynebacterium, Klebsiella pneumoniae and Candida albicans. The diagnosis is usually established from the clinical findings, though imaging techniques such as computed tomography are sometimes used, and treatment is based on broad spectrum antibiotic therapy and early surgical debridement.1,3
From 2013, when canagliflozin was approved by the FDA for the treatment of patients with DM2, until May 2019, a total of 55 cases of FG treated with SGLT2i were reported versus 19 cases in which patients received other types of glucose-lowering drugs, according to the FDA Adverse Event Reporting System (FAERS). Fournier's gangrene has been associated with all marketed SGLT2i except ertugliflozin (probably because the latter drug has been on the market for only a brief period). In the aforementioned 55 patients, the mean time from the start of treatment to the development of FG was 9 months. The most commonly used SGLT2i was canagliflozin. Three patients died, with no mention being made of any relation to previous urological manipulations.6
We conducted a PubMed search up until October 2019, and identified three clinical cases reporting a relationship between SGLT2i and the development of FG. Elshimy et al. reported a 57-year-old male treated with empagliflozin for 10 days who required two surgical interventions for the treatment of FG, with a favorable course.7 Onder et al. in turn described a 64-year-old male treated with dapagliflozin for 6 months, requiring three surgical interventions and a colostomy.8 Lastly, Kumar et al. reported a 41-year-old male treated with empagliflozin for 14 months who had undergone urological tract manipulations and required two surgical interventions.9 One study found the incidence of FG to be 15 cases/100,000 patients treated with SGLT2i.10
According to the Summary of Product Characteristics of dapagliflozin, this drug should not be started in patients with a GFR <60ml/min, and should be suspended if the GFR persistently falls to <45ml/min. Our patient had maintained stable renal function (GFR >60ml/min), but was at a high risk of impaired renal function due to a history of prostate cancer subjected to radiotherapy, right nephrectomy and the presence of a suprapubic catheter due to urethral stenosis. Closer monitoring of renal function is thus indicated, similar to that applicable to patients with a GFR <60ml/min (at least 2–4 times a year). In the event of a risk of volume depletion and/or arterial hypotension, certain recommendations apply: (1) caution should be exercised in patients for whom dapagliflozin-induced reductions in blood pressure may pose a risk, such as individuals subjected to antihypertensive treatment with a history of arterial hypotension; (2) in the case of intercurrent diseases that may lead to volume depletion, close monitoring of volume status and electrolytes is recommended; and (3) temporary discontinuation of dapagliflozin is advised in patients who develop volume depletion, until the latter has been corrected. The placement of a suprapubic catheter is not without complications, though their incidence is generally 1.6–2.4% and mainly involves urinary tract infections, hematuria, catheter blockage, skin infections and bladder lithiasis. The temporary discontinuation of dapagliflozin after suprapubic catheter placement may therefore be considered.
In conclusion, we report a case of FG in a patient with DM2 treated with dapagliflozin and presenting multiple predisposing factors. Although FG is an uncommon condition, the benefits of treatment with SGLT2i should be weighed in patients with risk factors for the development of the disease, particularly if they are to undergo urological manipulations of any kind.
The patient gave his informed consent to publication of the case.
Please cite this article as: García-García A, Galeano-Valle F, Nuevo-González JA, Demelo-Rodríguez P. Gangrena de Fournier e inhibidores de la SGLT2: a propósito de un caso. Endocrinol Diabetes Nutr. 2020;67:423–425.