Treatment with the MiniMed 640G-SmartGuard® system (640G-SG, sensor-augmented insulin pump system with low predicted glucose suspension feature) has been shown to decrease risk of hypoglycemia without altering metabolic control in patients with T1DM. The study purpose was to assess the impact of 640G-SG on hipoglycemia frequency and on metabolic control in a pediatric population with T1DM.
Patients/methodsA retrospective study on 21 children treated with 640G-SG. HbA1c, mean blood glucose (mg/dL), glucose variation coefficient, frequency of hypoglycemia (<70mg/dL) and hyperglycemia (>180mg/dL), daily capillary blood glucose measurements, ketosis/diabetic ketoacidosis, and severe hypoglycemic episodes were analyzed and compared before and during use of the system. Fasting blood glucose, frequency of sensor use and number and duration of system suspension events were also assessed in the last month of use of the system.
ResultsAll patients used the system continuously (5.0±2.1 months), with a median sensor use of 92%. Significant decreases were seen in hypoglycemia frequency (10.4±5.2% to 7.6±3.3%, p=0.044) and number of capillary blood glucose measurements (11.3±2.2 to 8.1±2.1, p<0.001), and there was no increase in hyperglycemia frequency (p=0.65). Mean system suspension time was 3.1±1.2h/day (37.3% of overnight stops). Changes in HbA1c, mean blood glucose, and variation coefficient were not significant. No patient experienced diabetic ketoacidosis or severe hypoglycemia.
ConclusionsThe sensor-augmented pump with the predictive low glucose suspension management system, as implemented in the 640G-SG system, can help avoid risk of hypoglycemia without significantly affecting metabolic control or causing diabetic ketoacidosis, and decrease the burden of additional capillary blood glucose measurements in our pediatric cohort.
El tratamiento con el sistema MiniMed 640G-SmartGuard® (640G-SG, infusión subcutánea continua de insulina con sensor de monitorización continua de glucosa intersticial implementado con suspensión automática por predicción de hipoglucemia) ha demostrado en estudios previos, disminución del riesgo de hipoglucemia sin producir alteraciones en el control metabólico en pacientes con DM1. El objetivo del estudio fue evaluar la efectividad del sistema 640G-SG sobre la frecuencia de hipoglucemia y su impacto sobre el control metabólico en una población pediátrica con DM1.
Pacientes/métodosEstudio retrospectivo que incluyó 21 niños tratados con 640G-SG. Se analizaron previo y durante su uso: HbA1c, glucemia media (mg/dl), coeficiente de variación de glucosa, frecuencia de hipoglucemia (<70mg/dl) e hiperglucemia (>180mg/dl), controles de glucemia capilar/día, episodios de cetosis/cetoacidosis e hipoglucemias graves. En el último mes de uso: glucemia en ayunas, frecuencia de uso del sensor y número y duración de eventos de suspensión.
ResultadosLos pacientes llevaron el sistema continuamente durante 5,0±2,1 meses con mediana de uso del 92%. Objetivamos disminución significativa de la frecuencia de hipoglucemia (10,4±5,2% a 7,6±3,3%, p=0,044) y del número de controles de glucemia capilar/día (11,3±2,2 a 8,1±2,1, p<0,001), sin aumento de hiperglucemia (p=0,65). Duración media de suspensión de infusión de insulina 3,1±1,2 h/día (37,3% suspensión nocturna). Sin cambios significativos en HbA1c, glucemia media, ni coeficiente de variación. Ningún paciente presentó cetosis/cetoacidosis ni hipoglucemia grave.
ConclusionesLa suspensión automática de infusión de insulina por predicción de hipoglucemia implementada en MiniMed 640G-SmartGuard® ayuda a evitar el riesgo de hipoglucemia, sin empeorar el control metabólico ni provocar cetosis/cetoacidosis, y reduce la carga de controles adicionales de glucemia en nuestra cohorte pediátrica.
Intensification of the treatment of type 1 diabetes mellitus (DM1) has been shown to improve metabolic control and to maintain pancreatic reserve for a longer period, with a resultant decrease in the risk of short- and middle-term complications.1,2 However, there is still concern regarding hypoglycemia, which may be caused by physical exercise, excessive insulin boluses, decreased food intake, etc., and currently constitutes a limiting factor for the introduction of intensive therapy. As is generally known, most of these events, in both the pediatric and adult populations, occur at night,3,4 and many of them are serious,5,6 causing great anxiety and compromising the quality of life of both parents and caregivers7 and the patients themselves. Furthermore, undiagnosed nocturnal hypoglycemic episodes may be the cause of subsequent inadvertent hypoglycemia, with a short and long-term impact on the nervous and cardiovascular systems.8,9
Continuous subcutaneous insulin infusion (CSII) therapy, combined with continuous interstitial glucose monitoring (CGM) with automatic suspension in the event of hypoglycemia, has been shown to be effective for reducing hypoglycemic events.10 This system automatically suspends insulin infusion for 2h when a given glucose level is reached, in order to shorten the duration of the event.11 This system makes it possible to shorten hypoglycemic episodes with no significant increase in hyperglycemia rates or the occurrence of ketosis.4
The next step toward the artificial pancreas has been the development of an integrated system (IS) consisting of CSII plus CGM, with the added feature of the automatic suspension of insulin infusion when low glucose is predicted, followed by the automatic resumption of infusion once this risk has been overcome. This allows for the prevention of the occurrence of hypoglycemic events and for shortening their duration if they should occur. The safety of this IS has been verified even in studies where only the nocturnal period was evaluated (due to the increased frequency of inadvertent hypoglycemia), in which the frequency of such events was decreased by up to 50%.12
This system is currently available in the MiniMed 640G-SmartGuard pump® ([640G-SG], Medtronic MiniMed, Northridge, CA, USA), with the “predictive low glucose suspension” feature, that suspends basal insulin infusion when the occurrence of hypoglycemia is predicted. This system has been evaluated in a limited number of adult and pediatric patients in studies involving hospital control where a significant decrease has been shown in hypoglycemic events with no increase in the frequency of hyperglycemia and no evidence of ketosis.13
The primary objective of this study was to assess the effectiveness of the 640G-SG system in terms of the risk of hypoglycemia, and its impact upon metabolic control in routine clinical practice, in a cohort of pediatric patients with T1DM.
Patients and methodsA retrospective, descriptive study was conducted in 21 pediatric patients with T1DM (43% males) monitored at the Pediatric Diabetes Unit of Ramón y Cajal University Hospital, who were treated with the MiniMed 640G-SmartGuard integrated system®, which includes the predictive low glucose suspension feature. The SmartGuard system automatically stops insulin infusion if the interstitial glucose level is predicted to drop to 20mg/dL above the pre-established threshold limit within 30min and the blood glucose level is 70mg/dL or less above the established limit. Insulin infusion automatically resumes when the interstitial glucose level is 20mg/dL above the pre-established threshold limit, is predicted to be 40mg/dL above that threshold within 30min, and infusion has been stopped for at least 30min (if infusion has not been manually resumed).
Patients were trained in the use of the 640G-SG system and the Contour® Next Link 2.4 glucometer (Bayer, Indianapolis, IN, USA) by diabetologists and educators from the Pediatric Diabetes Unit. They were also trained by qualified Medtronic staff to use the Enlite™ sensor (Medtronic) and Guardian™ 2 Link transmitter (Medtronic). At the start of the use of the 640G-SG system, a glucose level of 60mg/dL was established as the threshold limit for hypoglycemia for 24h. This was considered adequate for maximizing the effect of the predictive low glucose suspension function. In order to avoid hyperglycemia, parents/caregivers were advised to let the system operate without interference, except in the case of a lot of active insulin or previous strong exercise, or in the presence of trend arrows indicating a rapid decrease in glucose levels. This suspension threshold, while lower than those established in other studies (threshold levels of 70mg/dL14,15 and 80mg/dL12,16–18), was proposed in a recently published protocol.19 All the alarms were disabled, except for the hypoglycemia alarm, which is predefined by default, in order to minimize the risk of unnecessary interference by parents or caregivers. Patients measured their capillary blood glucose levels before making treatment decisions such as pre-intake or corrective bolus administration in situations of hypoglycemia or physical exercise.
Data were collected from clinical histories, glucometer downloads, and the Care Link® Pro Software (Medtronic) before the IS was started and at the time of analysis of the variables, coinciding with a scheduled clinical control.
The following comparisons were made before and after the use of the 640G-SG system (data of the last 90 days): glycosylated hemoglobin (normal value 5.31±0.31% [HbA1c, HPLC-Menarini]), mean blood glucose (mg/dL), glucose coefficient of variation (%, SD/mean blood glucose×100), frequency of hypoglycemia (blood glucose <70mg/dL) and hyperglycemia (blood glucose >180mg/dL) with respect to the total capillary blood glucose controls (CGCs) made in that period, and the daily number of CGCs. These data were downloaded from the glucometer, with the recommended minimum controls being taken as a reference.
The following parameters were also analyzed in the last 30 days of treatment: fasting blood glucose (mg/dL), the frequency of sensor use, and system suspension events, with their corresponding mean duration. Data were given as absolute and relative values, mean±standard deviation (SD), median, and range. The SPSS statistical package was used for data analysis, based on nonparametric and Chi-squared tests. Values of p<0.05 were considered statistically significant.
ResultsMean (±SD) patient age was 10.0±3.4 years (range: 2.4–16.3) at the time of the study, and 4.3±3.2 years (0.9–11.9) at the diagnosis of T1DM. The mean disease duration was therefore 5.7±2.9 years (0.7–11.0). With regard to treatment before the start of 640G-SG, 8 patients (33%) were already using CSII and some other type of CGM; 10 patients (48%) used CSII without CGM; and three patients (19%) who started to use the IS (MiniMed 640G-SmartGuard®) were simultaneously receiving multiple dose insulin. Only two of the 8 patients using CSII and CGM were equipped with automatic suspension of insulin infusion for hypoglycemia before the start of the study. The mean time of CSII use before starting 640G-SG was 5.3±2.9 years (0.2–10.4). None of the patients had a history of severe hypoglycemia or diabetic ketoacidosis. The indications of 640G-SG were: frequent (>10%) mild hypoglycemic episodes in 48% of the patients; inadvertent hypoglycemia in 19% (Clarke test in children over 8 years of age, n=17); and quality of life improvement in 33%. The duration of use of the system was 5.0±2.1 months (1.0–9.2), and 14.3% of the patients had been using it for less than three months at the time of data analysis. During this period, patients wore the system on a continuous basis, with a median use of the sensor of 92% (interquartile range: 81.5%–94%).
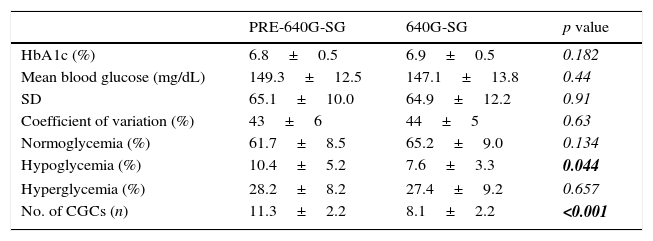
The primary outcome was a significant decrease in the rate of hypoglycemia (Table 1) from 10.4±5.2% to 7.6±3.3% (p=0.044) before and after use of the 640G-SG respectively, with no significant increase in the hyperglycemia rate (28.2±8.3% vs 27.4±9.2%; p=0.65). A significant decrease was also found in the number of CGCs: from 11.3±2.2 to 8.1±2.1 in 24h (p<0.001).
Metabolic parameters of T1DM control before and during the use of the integrated system.
| PRE-640G-SG | 640G-SG | p value | |
|---|---|---|---|
| HbA1c (%) | 6.8±0.5 | 6.9±0.5 | 0.182 |
| Mean blood glucose (mg/dL) | 149.3±12.5 | 147.1±13.8 | 0.44 |
| SD | 65.1±10.0 | 64.9±12.2 | 0.91 |
| Coefficient of variation (%) | 43±6 | 44±5 | 0.63 |
| Normoglycemia (%) | 61.7±8.5 | 65.2±9.0 | 0.134 |
| Hypoglycemia (%) | 10.4±5.2 | 7.6±3.3 | 0.044 |
| Hyperglycemia (%) | 28.2±8.2 | 27.4±9.2 | 0.657 |
| No. of CGCs (n) | 11.3±2.2 | 8.1±2.2 | <0.001 |
CGC: capillary glucose control; SD: standard deviation; HbA1c: glycosylated hemoglobin; 640G-SG: MiniMed 640G-SmartGuard.
The results in boldface indicate statistically significant values (p<0.05).
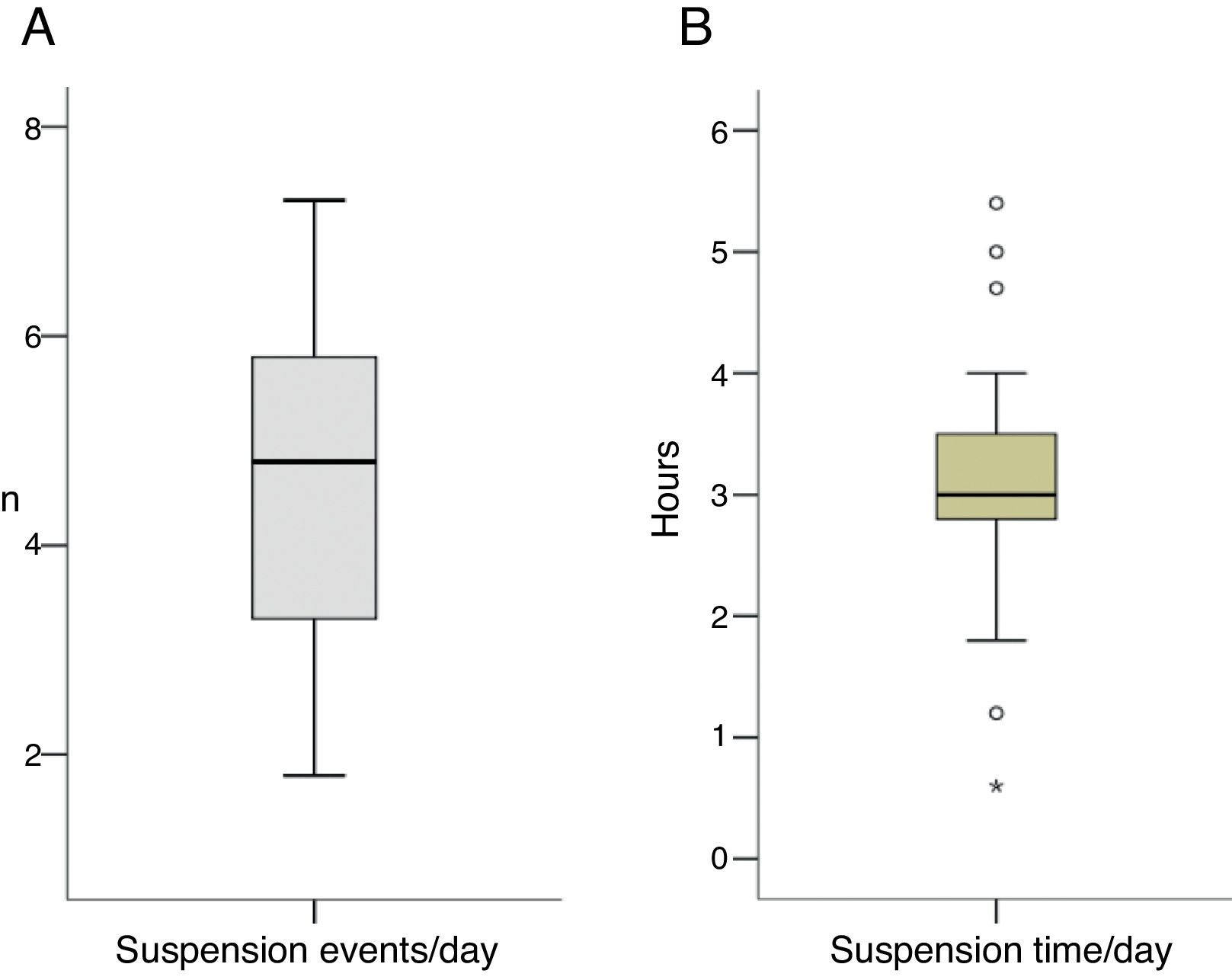
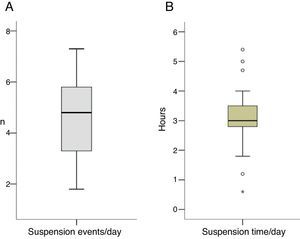
With regard to IS suspension, 4.6±1.6 suspension events (1.8–7.3) were recorded daily (40.4% of them at night), with a duration of suspension of 3.1±1.2h (0.6–5.4) daily (37.3% nocturnal suspension time) (Fig. 1A, B). Most suspensions lasting 2h were recorded at night (39% daytime suspensions vs 61% nocturnal suspensions). On the other hand, fasting blood glucose levels tended to decrease, but the difference did not reach statistical significance (p=0.16).
There were no significant differences between fasting blood glucose levels found following nocturnal IS suspension and without nocturnal suspension (138.9±14.6mg/dL vs 133.1±21.1mg/dL; p=0.11). Finally, no differences were found in HbA1c (6.8%±0.48% vs 6.9%±0.49%; p=0.18), mean blood glucose (149.3±12.5 vs 147.2±13.8mg/dL; p=0.44) or its coefficient of variation (CV) (43.1%±5.9% vs 43.8%±5.3%; p=0.64) when data recorded before and after the use of the IS were compared. No patient experienced episodes of diabetic ketoacidosis, ketosis, or hyperglycemia during the study period.
It should be mentioned that the 640G-SG system was removed from three patients due to family decision, with a mean time of use of the system of 2.41±0.78 months (1.07–3.77).
DiscussionNo data have yet been published regarding clinical experience with the MiniMed 640G-SmartGuard® in the Spanish pediatric population. The system has been available in Spain since April 2015. Since T1DM is one of the chronic diseases most relevant from asocial and healthcare perspective, the data reported suggest that the 640G-SG system is able to significantly decrease the risk of hypoglycemic events in the short term. Risk reduction in our study was 26.9% (from 10.4% to 7.6%). Good system tolerability and adherence were also found, as shown by the sustained use of CGM in 92% of the cases.
The current literature on the effectiveness of this system includes the Choudhary et al. study,13 involving 16 pediatric patients, which reported results consistent with our own findings in terms of the prevention of hypoglycemic events, with no associated increase in the hyperglycemia rate. Biester et al., in a recent study, also evaluated this system in children and adolescents (2 weeks with CSII and sensor vs 6 weeks with the IS), and concluded that this automatic predictive low glucose suspension algorithm is safe for avoiding such episodes in this population with no associated worsening of metabolic control in terms of HbA1c.15 Similarly, recent studies have also reported decreased hypoglycemia rates20 and improved quality of life.21
On the other hand, the PILGRIM study,14 which divided the patients into three groups and used a computerized model to compare a similar system (CSII Paradigm®VEO with the Enlite blood glucose sensor™ [Medtronic, Inc.]) with automatic suspension for hypoglycemia and a control group (no CSII suspension), found a significant decrease in the number of hypoglycemic events in 22 patients aged 14–20 years following physical exercise as a glucose-lowering stimulus. The hypoglycemia rate decreased by 26.7% compared to non-suspension of the pump, and by 5.3% versus automatic suspension for hypoglycemia. A significant decrease in the duration of hypoglycemic events as compared to automatic suspension was also seen.
An evaluation in the hospital setting of a similar system (Medtronic Paradigm Veo CSII™ with the Enlite™ blood glucose sensor) versus the administration of extra insulin boluses with elevation of the basal levels by up to 180% during the nighttime period revealed a significant decrease in the need to administer carbohydrates as treatment for hypoglycemia in patients who used the system with predictive low glucose suspension. Hyperglycemia was not recorded after pump suspension, even when the suspension lasted 2h.16
A significant decrease was seen in the number of CGCs, from 11.3±2.2 to 8.1±2.1, but patients nevertheless continued to perform the minimum CGCs required for adequate metabolic control, thus indicating an improved quality of life, as has already been suggested in earlier studies.22
Continuous use of the system is not associated with a poorer evolution of metabolic control in terms of HbA1c, overall mean blood glucose and coefficient of variation, as evidenced by a number of studies where the variations were not found to be significant.13,17 A slight improvement in mean blood glucose levels was seen in our study after using the IS, but did not reach statistical significance.
One of the main disadvantages of suspending insulin infusion for long periods of time is the risk of diabetic ketoacidosis. In this regard, a study evaluated the risk of ketosis after prolonged suspension of the IS, even with normal blood glucose values, and reported no significant changes in fasting β-hydroxybutyrate levels after nighttime suspension. This suggests that routine ketone body measurements are not required, and that indications for such tests should be no different from those applicable to patients receiving conventional CSII treatment.18 This had already been shown in earlier studies with low glucose suspension systems, in which no ketonuria or ketonemia episodes were recorded after continuous suspension of the system for 2h.10,23 No patient in our study reported ketosis episodes while using the system.
As regards suspension events and their mean daily duration, 4.6±1.6 suspension events were recorded on average in our study in 24h (1.8–7.3). Of these, 40.4% were nocturnal events, with a mean daily suspension time of 3.1±1.2h (0.6–5.4), of which 37.3% corresponded to the nighttime period. This is consistent with the results reported by Choudhary et al.13 in a cohort of patients aged 31.7±17.1 years where daytime suspension events were more frequent. However, these authors recorded shorter event duration. In addition, they reported a mean system suspension time of 56.4±9.6min, which is shorter than in our study. The difference may be explained by the younger age of our population. As previously noted, studies have reported that most hypoglycemic events usually occur during the night. A study conducted on a pediatric population (4–14 years of age) published in 2015 evaluated whether the MiniMed Paradigm REAL-Time Veo system with the Enlite™ blood glucose sensor (Medtronic, Inc.) shortened the hypoglycemia time during the night. The authors found significant reductions in hypoglycemia time by 54% and 50% in children aged 11–14 and 4–10 years respectively, while no differences were found in ketosis events.17 Fasting blood glucose levels in patients with the active IS versus the inactive IS (controls) were also compared, and no significant differences were found in the 4–10 years age group (158±22mg/dL vs 154±25mg/dL respectively; p=0.11). This agrees with the results of our study (fasting blood glucose after nocturnal suspension of the IS, 138.9±14.6mg/dL vs 133.1±21.1mg/dL without IS suspension; p=0.11). However, the mentioned study found significant differences in the 11–14 years age group (176±28mg/dL vs 159±29mg/dL respectively; p<0.001), in agreement with the results reported by Sherr et al.,23 who assessed the system with low glucose suspension and recorded significantly greater fasting blood glucose levels in system users as compared to controls (191±68mg/dL vs 141±75mg/dL respectively; p<0.0001).
Because of its retrospective design, one of the main limitations of this study was the impossibility of performing blinded CGM before the start of treatment to ensure greater reliability when comparing the data downloaded from the IS referring to the frequency, duration, and severity of hypoglycemic events, and the frequency of hyperglycemia. For this same reason, there could be difficulties in comparing data on the frequency of symptomatic and asymptomatic hypoglycemia, of which the latter mostly occur during the nocturnal period. This is why the two time periods were analyzed separately. On the other hand, since the CGM system was operating in real time, these patients might not have confirmed the hypoglycemic values of the sensor with CGCs. The frequency of hypoglycemia following the use of the IS may therefore have been underestimated. However, despite the decrease in CGCs, these were still sufficient in number for adequate metabolic control. On the other hand, one of the factors that could condition higher fasting blood glucose levels, and which was taken into consideration in our study, is the fact that patients or parents do not always follow the therapeutic approach when faced with a given hypoglycemic episode, and over correction may have occurred, with an unnecessary administration of carbohydrates in cases where the patient could have remained under normoglycemic conditions and the IS simply “allowed to do its job”. A further limitation, which it might be worth taking into account in future prospective studies, concerns the use of quality of life questionnaires, the fear of hypoglycemia surveys for parents and patients, and treatment satisfaction surveys. Nevertheless, a significant decrease in the number of CGCs (from 11.3±2.2 to 8.1±21 controls a day), and a sensor use frequency greater than 90% in these patients are indirect signs of good acceptance.
In conclusion, the use of CSII with CGM and the added feature of predictive low glucose suspension (MiniMed 640G-SmartGuard®) helps prevent the occurrence of hypoglycemic events with no significant increase in hyperglycemia. On the other hand, no significant changes are seen in fasting blood glucose levels, and no diabetic ketoacidosis, ketosis, or severe hypoglycemia occur in standard clinical practice.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Villafuerte Quispe B, Martín Frías M, Roldán Martín MB, Yelmo Valverde R, Álvarez Gómez MÁ, Barrio Castellanos R. Efectividad del sistema MiniMed 640G con SmartGuard® para la prevención de hipoglucemia en pacientes pediátricos con diabetes mellitus tipo 1. Endocrinol Nutr. 2017;64:198–203.