Consumptive hypothyroidism is a very uncommon syndrome attributable to the increased activity of type 3 thyronine deiodinase (D3) of tumor origin. The half-life of this enzyme is 12 h, and it mediates the conversion of tetraiodothyronine (T4) into reverse triiodothyronine (T3r) and triiodothyronine (T3) into diiodotyrosine (T2), both inactive forms. This in turn stimulates a positive feedback at pituitary gland level, incrementing the levels of thyroid stimulating hormone (TSH), which stimulates thyroid hormone production.1 The enzyme is physiologically expressed in placental tissue, uterine endometrium and the central nervous system, but tumors overexpressing D3 exceed the secretory capacity of the thyroid gland, giving rise to consumptive hypothyroidism.2
We present a case of consumptive hypothyroidism in a patient with prior primary autoimmune hypothyroidism.
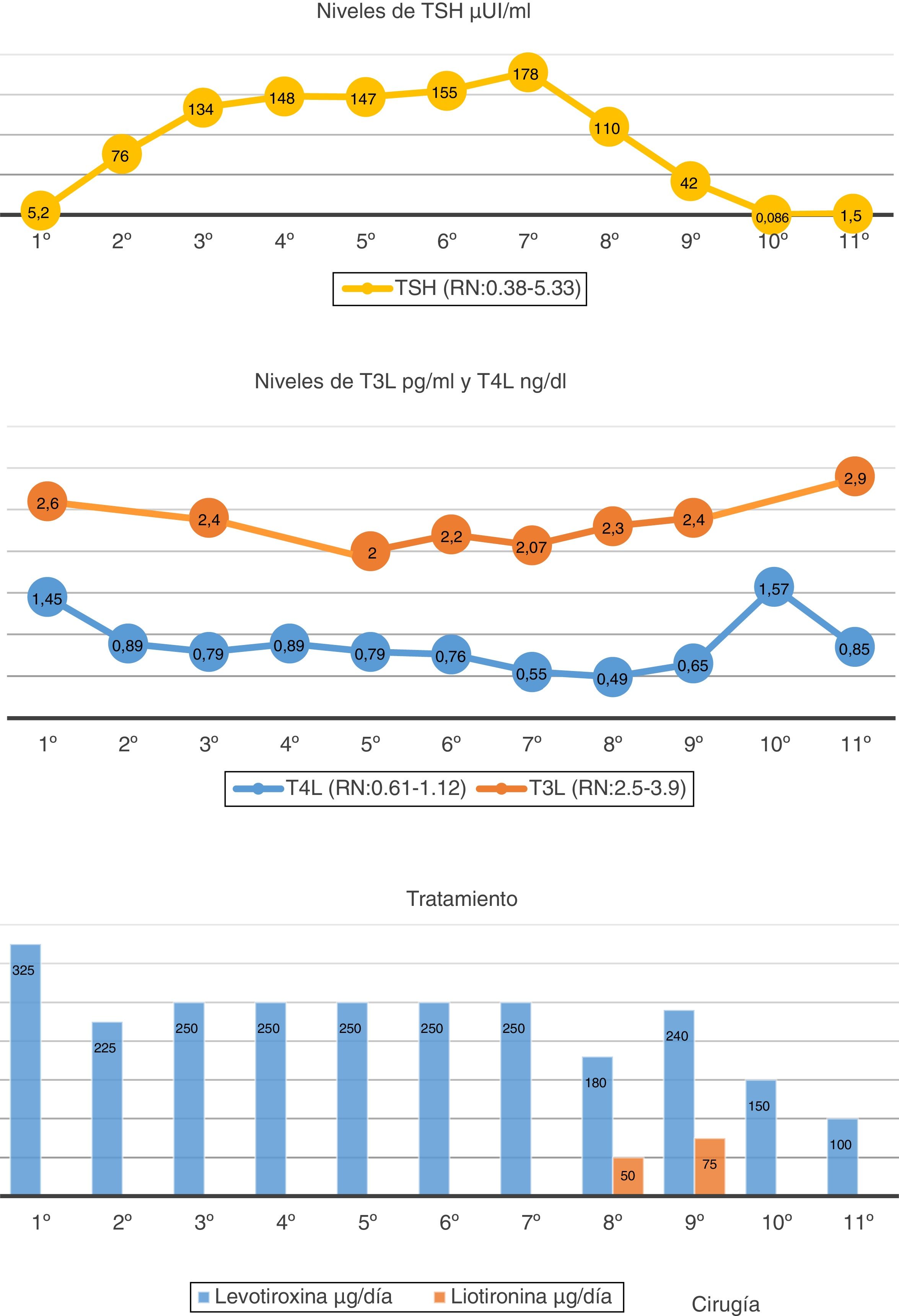
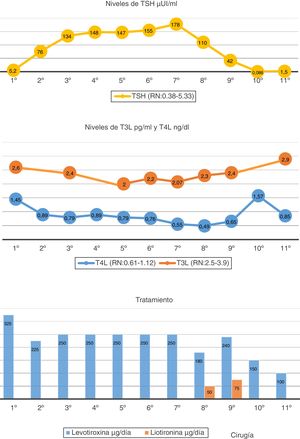
A 64-year-old woman with no relevant personal or family history reported to Endocrinology due to difficult-to-control primary hypothyroidism. The case history revealed primary autoimmune hypothyroidism diagnosed 15 years previously and routinely treated with levothyroxine 125 μg/day. Over the past two years the dose had been gradually increased to 325 μg/day. The initial laboratory tests in Endocrinology revealed: TSH: 5.2 (normal range [NR]: 0.38-5.33) μIU/mL; FT4: 1.45 (NR: 0.61-1.12 ng/ml: FT3: 2.6 (NR: 2.5-3.9) pg/mL; TPO antibodies: 183 (NR: 0-9) IU/mL. At the initial visit, the patient was asymptomatic except for discrete asthenia. The physical examination revealed no pathological findings except for grade 2 goiter, without nodules.
Since the FT4 levels were initially elevated, the levothyroxine dose was reduced from 325 to 225 μg/day. The TSH levels reached 134 μIU/mL; the decision was therefore made to increase the dose to 250 μg/day. Despite the high levothyroxine doses, TSH remained very high. The diagnostic assessment was therefore continued. The evolution over time of the hormone levels and the levothyroxine dose are summarized in Fig. 1.
The patient claimed good adherence to therapy, with no treatment capable of altering levothyroxine absorption. The tests requested to discard malabsorption-related diseases proved negative (Helicobacter pylori, celiac disease and chronic gastritis). Interference with the laboratory method was ruled out, with the same thyroid hormone results being obtained by measurements using different techniques. The presence of macro-TSH was ruled out by the polyethylene glycol (PEG) precipitation method. Although the peripheral hormones were within the normal/low range, given the progressive increase in TSH levels, we considered the possibility of a TSH-producing tumor (TSHoma) coexisting with background primary hypothyroidism. The pituitary gland magnetic resonance imaging (MRI) study revealed a 3 mm microadenoma. A TRH stimulation test was performed following the suspension of levothyroxine: Basal TSH measurement: 303.59 IU/l, FT4: 0.19 ng/dl and FT3: 1.76 pg/mL. TSH levels at 30 min: 391.1, at 60 min: 406.6, at 90 min: 333.6, and at 120 min: 302.1 IU/l. The stimulus test result proved normal, leading us to discard the presence of a TSHoma. The rest of the pituitary profile was normal.
Over time, the patient started to experience pain in the left flank region. Ultrasound and then an abdominal computed tomography (CT) scan were therefore requested. A 23 cm well-defined, polylobular, heterogeneous and highly vascularized peritoneal mass displacing adjacent structures, without infiltration was identified. With the suspicion of consumptive hypothyroidism, the treatment was modified, liothyronine sodium (Cynomel®) 25 μg one tablet every 8 h and liquid levothyroxine being added to facilitate absorption (levothyroxine Serb®) 240 µg/day. The determination of reverse T3 was requested, with a result of 1.28 ng/mL (adult NR: 0.09-0.35), thus confirming the diagnostic suspicion. A biopsy was performed, revealing a fusocellular mesenchymal neoplasm with a hemangiopericytoma pattern and an adipocytic component. The intraabdominal tumor was removed without complications. The pathology report described a well-delimited, solitary fibrous fat-producing tumor measuring 23 cm in size and weighing 3050 g, with no vascular invasion. After surgery, the levothyroxine dose had to be reduced to 100 μg/day, with equilibrium being achieved in the thyroid function tests at this dose. The patient is currently asymptomatic and without evidence of tumor relapse.
Consumptive hypothyroidism is a rare condition mainly reported in children with hepatic hemangiomas and hemangioendotheliomas characterized by tumor overexpression of type 3 thyronine deiodinase 3 (D3).3 This enzyme inactivates T4 and T3, converting them to reverse T3 and T2, respectively, stimulating TSH through positive feedback. In patients with a competent thyroid gland, partial compensation may occur through increased hormone production. In patients with background primary hypothyroidism, severe hypothyroidism is induced, requiring the treatment to be increased to supraphysiological doses. In most cases, levothyroxine and liothyronine should be combined. In adults, only 7 cases of consumptive hypothyroidism were found in the literature4: one hepatic hemangioma and one hemangioendothelioma,5,6 three GIST tumors,7–9 and two fibrous tumors.1,10
Solitary fibrous tumor is an uncommon soft tissue neoplasm originating from fibroblasts, and usually presents as a well-defined, solid and hypervascularized abdominal mass. The tumor is slow-growing and asymptomatic; the diagnosis is therefore commonly established due to the compression of neighbouring organs. Treatment is surgical, and the prognosis is usually good. However, 15–20% of all cases may exhibit malignant behavior, with local recurrences or distant metastases. Follow-up for at least 10 years is therefore recommended.
Consumptive hypothyroidism should be suspected in patients with elevated TSH, FT4 and FT3 in the normal/low range, and elevated reverse T3, and who require treatment with exceptionally high doses of thyroid hormone. Treatment of this condition is achieved by acting upon the tumor that produces it. In children with consumptive hypothyroidism, it is more common to find vascular tumors that respond well to medical treatment. Solid tumors are more common in adults, and surgical treatment is the treatment of choice in such cases.
In conclusion, consumptive hypothyroidism can be considered as a rare paraneoplastic syndrome, which sometimes occurs as the first sign of the tumor, especially in asymptomatic neoplasms such as fibrous tumors. This, therefore, should be taken into account in the differential diagnosis of hypothyroidism.
Please cite this article as: Civantos Modino S, Pacheco Delgado MS, Martínez-Piñeiro Muñoz JA, Cancer Minchot E, Cánovas Molina G, Rodríguez Robles A. Hipotiroidismo consuntivo en paciente con hipotiroidismo primario previo. Endocrinol Diabetes Nutr. 2021;68:76–78.