Under physical exercise conditions, muscles can synthetise and release myokines and these molecules can exert paracrine and endocrine actions. Females with obesity have a sedentary lifestyle with alterations in myokine levels.
ObjectiveThe aim of our study was to evaluate the effect of physical exercise on myokine levels, anthropometric parameters, clinical data, impedance parameters, and muscle ultrasound data in sedentary females with obesity.
Material and methodsAnthropometric data, muscle mass by ultrasound at the quadriceps level, myokine determination, and blood pressure were collected at baseline and after 12 weeks in 25 females with obesity. For 12 weeks, the physical exercise programme was prescribed through an online platform.
ResultsAfter the physical exercise programme, there was a significant improvement in body mass index (−1.49±0.1kg/m2; p=0.02), weight (−3.9±0.7kg; p=0.01), waist circumference (−7.2±0.2cm; p=0.01), skeletal muscle mass (5.4±1.2kg; p=0.01), appendicular skeletal muscle mass index (0.5±0.1kg; p=0.02) and appendicular skeletal muscle mass (1.4±0.1kg; p=0.03), and a decrease in fat mass (−4.1±0.2kg; p=0.01) and blood pressure. The ultrasound parameters of the anterior rectus quadriceps muscle improved significantly. The following biochemical parameters decreased; insulin levels (−66.3±10.2pg/ml; p=0.04), HOMA-IR (−0.4±0.1 units; p=0.03), apelin (−3.5±0.2IU/l; p=0.04), FABP3 (−143.6±38.1pg/ml; p=0.03), IL6 (−4.1±0.02pg/ml; p=0.02), myostatin (−81.6±18.1pg/ml; p=0.04), and FGF21 (−9.5±1.1pg/ml; p=0.03).
ConclusionThe prescription of physical exercise with an online platform for females with obesity decreases weight, body fat mass and increases muscle mass, producing a decrease in insulin resistance and some myokine levels.
En condiciones de ejercicio físico, los músculos pueden sintetizar y liberar mioquinas y estas moléculas pueden ejercer sus acciones paracrinas y endocrinas. Las mujeres con obesidad tienen un estilo de vida sedentario con alteraciones en los niveles de mioquinas.
ObjetivoEl objetivo de nuestro estudio fue evaluar el efecto del ejercicio físico sobre los niveles de mioquinas, parámetros antropométricos, clínicos, de impedancia y de ultrasonido muscular en mujeres sedentarias con obesidad.
Material y métodosSe recogieron datos antropométricos, masa muscular por ecografía a nivel de cuádriceps, determinación de mioquinas, presión arterial al inicio y a las 12 semanas en 25 mujeres con obesidad. Durante 12 semanas se prescribió el programa de ejercicio físico a través de una plataforma online.
ResultadosDespués del programa de ejercicio físico, hubo una mejoría significativa en el índice de masa corporal (−1,49±0,1kg/m2; p=0,02), peso (−3,9±/0,7kg; p=0,01), circunferencia de la cintura (−7,2±0,2cm; p=0,01), masa muscular esquelética (5,4±1,2kg; p=0,01), índice de masa muscular esquelética apendicular (0,5±0,1kg; p=0,02) y masa muscular esquelética apendicular masa muscular (1,4±0,1kg; p=0,03). Y una disminución de la masa grasa (−4,1±0,2kg; p=0,01) y de la presión arterial. Los parámetros ecográficos del músculo recto anterior del cuádriceps mejoraron significativamente. Los siguientes parámetros bioquímicos disminuyeron; niveles de insulina (−66,3±10,2pg/mL; p=0,04), HOMA-IR (−0,4±0,1 unidades; p=0,03), apelina (−3,5±0,2UI/L; p=0,04), FABP3 (−143,6±38,1pg/mL; p=0,03), IL6 (−4,1±0,02pg/mL; p=0,02), miostatina (−81,6±18,1pg/mL; p=0,04), y FGF21 (−9,5±1,1pg/mL; p=0,03).
ConclusiónLa prescripción de ejercicio físico con una plataforma online para mujeres con obesidad disminuye el peso, la masa grasa corporal y aumenta la masa muscular, produciendo una disminución de la resistencia a la insulina y algunos niveles de mioquinas.
The prevalence of obesity worldwide has increased considerably in recent years.1 Obesity is a risk factor for developing cardiovascular events, osteoarthropathy, cancer and other well-known comorbidities.2 On the other hand, it is common to find a decrease in muscle mass in patients with obesity, which we call sarcopenia; this entity is known as sarcopenic obesity.3 Females have some barriers to realize physical activity and this fact is a potential driver to increase fat mass and loss of muscle mass. Patients with these two entities have a higher risk of metabolic disorders, and a higher prevalence of cardiovascular diseases and mortality rates.4 Regarding the approach to obesity through physical exercise, the World Health Organization (WHO) recommends that adults aged 18–64 years should perform at least 150min of moderate-intensity aerobic physical activity during the week or at least 75min of vigorous-intensity aerobic physical activity during the week.5 Different types of physical exercise, such as treadmill running, resistance exercise, and swimming have demonstrated health benefits.6 Nonetheless, during the ongoing COVID-19 pandemic, home-based physical activity programmes supported by digital solutions are commonly used to maintain a good weight balance.7 One of the barriers to performing physical exercise detected is the investment of time by the subject.8 For this reason the use of online platforms that allow physical exercise in the patient's own environment are showing good acceptance by the patient or user.9
On the other hand, muscle is the most important protein reserve in the human body. Furthermore, skeletal muscle is capable of producing and secreting different molecules in order to communicate with other tissues and of releasing endocrine, paracrine, and autocrine factors named myokines. These molecules show potentially important effects on non-muscle tissue and could provide cross-talk between muscle and adipose tissue.10 Both muscle disuse and exercise could lead to numerous physiological changes by reducing or augmenting the production of myokines. Under physical exercise conditions, muscles can synthetise and release myokines and these molecules can exert paracrine and endocrine actions. Physical exercise has positive effects on the balance of pro- and anti-inflammatory mediators, and the increase of pro-inflammatory markers is related to a sedentary life. A vicious circle can start, inflammation enhances accumulation of fat mass and enhances sarcopenia, reducing body strength and favouring sedentarism.
Accordingly, the aim of the current study was to understand the effect of physical exercise on myokine levels, anthropometric parameters, clinical data, impedance parameters, and muscle ultrasound data in sedentary females with obesity in a real-world study.
Material and methodsWe enrolled twenty-five inactive, obese (body mass index ≥30kg/m2) females who attended consultations at our hospital to assess their obesity. All patients signed an informed consent for their inclusion before participating in the study. The protocol was approved by the Bioethical Committee of the Health Area of HCUVa (PI20/2062) and the study was conducted in accordance with the Declaration of Helsinki.
The inclusion criteria of the patients were the presence of obesity diagnosed with a body mass index ≥30kg/m2, functional autonomy, physical inactivity (less than 30min of physical activity per week) as well as the habitual use by the patient in their daily life of a computer/tablet/mobile phone to access information. The exclusion criteria were the following; history of cardiovascular events (stroke or myocardial infarction); alcohol habit; active smoking habit; active oncological process; taking, during the 6 months prior to the study, drugs that influence lipid or glucose levels or other chronic medications; and that they had followed a hypocaloric diet during this period.
Body composition analyses were performed and blood samples were collected at baseline and after 12 weeks with the online exercise protocol. During the baseline visit, anthropometric data were collected (weight, height, body mass index (BMI), fat mass by impedance and waist circumference), muscle mass by ultrasound at the level of the rectus femoris quadriceps (RFQ) and blood pressure. To determine the biochemical parameters, 5ml of venous blood was aliquoted into tubes coated with ethylenediaminetetraacetic acid EDTA after an overnight fast of 10h. The following parameters were measured; insulin, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, C-reactive protein, Apelin (APLN), Fatty Acid-Binding Protein 3 (FABP3), Fibroblast Growth Factor 21 (FGF21), IL-6, IL-15, Irisin, Leukaemia Inhibitory Factor (LIF), Myostatin (MSTN)/GDF8, Osteocrin/Musclin (OSTN) and Osteonectin (SPARC).
The presence of metabolic syndrome (MS) was defined according to the criteria established by the Adult Treatment Panel III (ATPIII). Patients had to meet at least 3 of the following criteria to be diagnosed with MS; elevated fasting glucose or treatment for diabetes, elevated triglycerides (>150mg/dl) or treatment for dyslipidaemia, low HDL cholesterol<40mg/dl (men) or <50mg/dl (women), systolic blood pressure or elevated diastolic blood pressure (>130/85mmHg or antihypertensive treatment) and increased waist circumference (>94cm (men) or >80cm (women)). The month before inclusion in the physical exercise programme, the patients received instructions to follow standard dietary recommendations with 1600 calories per day, 80g of protein, 70g of fat with 50% monounsaturated fat, and 160g of carbohydrates and 15g of fibre, to avoid dietary changes throughout the physical exercise programme.
Physical exercise programme through an online platformThe physical exercise programme carried out by the subjects is conducted by registering on the web platform www.vibraup.com, either on a mobile device, tablet or computer. The patients made this record using an access code provided by their investigator through the web platform itself (email). The training programme lasted 12 weeks. Each subject performed 3 levels of 4 weeks duration and a weekly frequency of 2 days of training directed by the web platform; the training lasted between approximately 10 and 30min, varying depending on the patient's level (Supplementary material). Supplementary material reflects, as an example, 4 weeks of one level of this training programme, which consisted of performing a series of multi-joint exercises that combined strength exercises with cardiovascular exercises, called concurrent training. In this concurrent training, an increasing linear progression of both volume and intensity is proposed. The exercises carried out have been proposed based on a traditional organisation in the first levels, progressing to a circuit format in later levels where exercises with different objectives are alternated (strength, stabilisation, cardiovascular). Additionally, the subjects had a task based on increasing their physical activity, specifically climbing stairs and walking. The subjects were also asked to record their physical activity daily on the platform itself (floors climbed measured by the subject herself and steps taken measured using the G-Step mobile application (Green Health Care, LA, CA, USA)). Both investigator and patient had access to the web platform, where they could check their progress and daily data records, and thus learn about the evolution and adherence to the exercise programme.
Assessment of physical conditionParticipants were instructed to self-assess two strength tests directed by the web platform, so that on week 1, day 1 (which coincided with the start of the level) the subject would measure the following parameters: upper body strength (maximum number of push-ups in 30s), lower body strength (maximum number of squats in 30s) and walking 1500m in the shortest time possible. The basal and postintervention measurements were supervised and carried out by the patient together with one of the research professionals, ensuring understanding, correct execution and the way to record the results obtained from them for subsequent months.
Anthropometric parameters, muscle ultrasound and blood pressureHeight (cm) and waist circumference (cm) were measured with a non-elastic tape measure (Omrom, LA, CA, USA). Body weight was determined with the subjects without clothes, using a digital scale (Omrom, LA, CA, USA). Using these parameters, body mass index (BMI) (body weight (kg) divided by the square of height (m)) was calculated. The bioimpedance (BIA) measured the two components of impedance (Z); resistance (R) and the capacitance component (X). The Phase angle (PhA) is derived for the next equation PhA=(X/R)×(180°/π). The BIA provided data regarding fat mass (FM), skeletal muscle mass (SMM), appendicular muscle mass (aSMM), appendicular muscle mass index (aSMMI) as SMMI divided by squared height (EFG BIA 101 Anniversary, Akern, It).
All subjects underwent muscle ultrasound of the rectus femoris of the quadriceps of the left and right lower extremities with a 10–12MHz probe and a multifrequency linear array (Mindray Z60, Madrid, Spain). The probe was aligned perpendicular to the longitudinal and transverse axis of the rectus femoris quadriceps (RFQ); the evaluation was performed without compression at the level of the lower third from the superior pole of the patella and the anterosuperior iliac spine, measuring the area, circumference and the anteroposterior diameter. Echo intensity was assessed considering the whole muscle longitudinal section as region of interest (ROI) in the same above-mentioned area of RFQ. The grey scale spreads from 0 (black) to 255 (white). All ultrasound parameters were standardised dividing by the patient's height squared.
Mean systolic and diastolic blood pressures were calculated by averaging three consecutive measurements (Omrom, LA, CA, USA), after the subjects sat for 10min.
Biochemical parametersTo assess the lipid profile, we determined total cholesterol, HDL cholesterol, and triglyceride levels using the COBAS INTEGRA 400 analyser (Roche Diagnostic, Montreal, Canada). LDL cholesterol was calculated using the Friedewald formula (LDL cholesterol=total cholesterol-HDL cholesterol-triglycerides/5). Glucose levels were determined by an automated hexokinase oxidase method, and insulin was measured by electrochemiluminescence assay with the COBAS INTEGRA 400 analyser (Roche Diagnostic, Montreal, Canada). To calculate insulin resistance, the Homeostasis Model assessment (HOMA-IR) was used and calculated using these values (glucose×insulin/22.5). C-reactive protein (CRP) was measured by immunoturbimetry (Roche Diagnostics GmbH, Mannheim, Germany).
For myokines (Apelin (APLN), Fatty Acid-Binding Protein 3 (FABP3), Fibroblast Growth Factor 21 (FGF21), IL-6, IL-15, Irisin, Leukaemia Inhibitory Factor (LIF), Myostatin (MSTN)/GDF8, Osteocrin/Musclin (OSTN) and Osteonectin (SPAR)), we used the commercial kit: MILLIPLEX® Human Myokine Magnetic Bead Panel (HCYTOMAG-56K, EMD Millipore Corporation, MA, USA), for which we followed the manufacturer's instructions (according to the instructions of the manufacturer).
Statistical analysisStatistical analysis was performed with SPSS statistical software for Windows version 23.0 (SPSS Inc. Chicago, IL). The sample size was determined to detect differences of 2.5kg of body weight after the intervention with 90% power and 5% significance. The Bonferroni test was applied for multiple tests to reduce the type I error in the association analysis. Descriptive statistics for all variable values are presented as mean and standard deviation for continuous variables and as percentage for categorical variables. The variables were analysed with the ANOVA test with the Bonferroni post hoc test and the Student's t-test (for the variable with normal distribution) or the Kruskal–Wallis test (for the variable with non-normal distribution). The Chi-square test was used to assess the qualitative variables. p values below 0.05 were considered statistically significant.
ResultsIn total, 25 females with obesity were included with a mean age of 45.6±2.4 years. The mean body mass index was 32.9±1.6kg/m2 with a mean weight of 85.4±11.6kg. In the self-registration of 3 activities, it was detected that after the physical exercise programme, squats in 30s (17.7±2.9 vs 22.6±1.2; p=0.02), dips in 30s (15.9±3.5 vs 19.7±3.1; p=0.01) and the time in seconds needed to travel 1.5km (995.7±45.9 vs 833.4±35.5; p=0.01) significantly improved after the 12-week physical exercise programme.
Table 1 shows the evolution of the anthropometric variables and bioimpedance measurements after the intervention with physical exercise. Regarding the classic anthropometric variables, there was a significant decrease in BMI (−1.49±0.1kg/m2; p=0.02), weight (−3.9±0.7kg; p=0.01) and waist circumference (−7.2±0.2cm; p=0.01). Regarding the bioimpedance measurement variables, there was a significant decrease in fat mass (−4.1±0.2kg; p=0.01), an increase in skeletal muscle mass (SMM) (5.4±1.2kg; p=0.01), appendicular skeletal muscle mass index (aSMMI) (0.5±0.1kg; p=0.02) and appendicular skeletal muscle mass (aSMM) (1.4±0.1kg; p=0.03). Systolic (−5.8±0.7mmHg; p=0.02) and diastolic (−5.2±0.5mmHg; p=0.01) blood pressures also decreased significantly.
Adiposity and impedance parameters with blood pressure (mean±standard deviation).
| Baseline | 12 weeks | p | |
|---|---|---|---|
| Weight (kg) | 85.4±11.6 | 81.5±12.1* | 0.01 |
| Body mass index (kg/m2) | 32.9±1.6 | 31.4±4.6* | 0.02 |
| Waist circumference | 104.8±15.6 | 97.6±8.6* | 0.01 |
| Phase angle (°) | 6.2±0.8 | 6.1±0.7 | 0.59 |
| Fat mass (kg) | 35.4±9.2 | 31.0±8.6* | 0.01 |
| SMM (kg) | 22.4±2.1 | 27.8±2.2* | 0.01 |
| aSMM | 19.8±0.9 | 21.3±0.3 | 0.43 |
| aSMMI | 8.4±0.1 | 8.9±0.2 | 0.02 |
| SBP (mmHg) | 132.1±16.1 | 127.9±12.7* | 0.02 |
| DBP (mmHg) | 84.3±5.7 | 79.1±4.3* | 0.01 |
The BIA provided data regarding fat mass (FM), skeletal muscle mass (SMM), appendicular muscle mass (aSMM), skeletal muscle mass index (SMMI) as SMMI divided by squared height, appendicular muscle mass index (aSMMI) as SMI divided by squared height. SBP, systolic blood pressure; DBP, diastolic blood pressure.
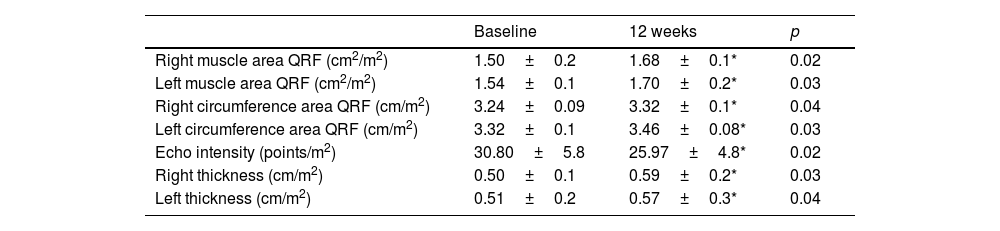
Table 2 shows the evolution of the ultrasound parameters of the quadriceps rectus femoris, with a significant increase in muscle area, muscle circumference and the Y axis of this muscle, both in the right and left lower extremities. Echo intensity decreased in a significant way, too.
Ultrasound parameters (mean±standard deviation).
| Baseline | 12 weeks | p | |
|---|---|---|---|
| Right muscle area QRF (cm2/m2) | 1.50±0.2 | 1.68±0.1* | 0.02 |
| Left muscle area QRF (cm2/m2) | 1.54±0.1 | 1.70±0.2* | 0.03 |
| Right circumference area QRF (cm/m2) | 3.24±0.09 | 3.32±0.1* | 0.04 |
| Left circumference area QRF (cm/m2) | 3.32±0.1 | 3.46±0.08* | 0.03 |
| Echo intensity (points/m2) | 30.80±5.8 | 25.97±4.8* | 0.02 |
| Right thickness (cm/m2) | 0.50±0.1 | 0.59±0.2* | 0.03 |
| Left thickness (cm/m2) | 0.51±0.2 | 0.57±0.3* | 0.04 |
QRF quadriceps rectus femoris. Ultrasound parameters were standardised dividing by the patient's height squared; Y axis muscle thickness, circumference, cross-sectional area and the mean grey value (MGV) (Echo intensity).
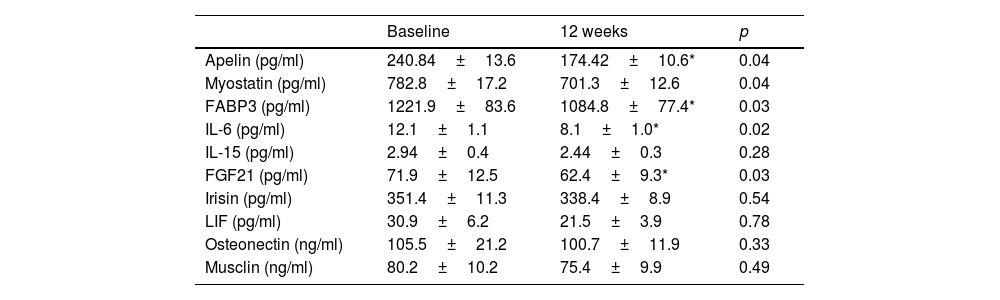
Regarding biochemical variables, Table 3 shows a significant decrease in insulin levels (−66.3±10.2pg/ml; p=0.04) and HOMA-IR (−0.4±0.1 units; p=0.03), the remaining parameters did not change significantly. The modifications of the different myokines are shown in table 4. There was a significant decrease in apelin levels (−3.5±0.2IU/l; p=0.04), FABP3 (−143.6±38.1pg/ml; p=0.03), IL6 (−4.1±0.02pg/ml; p=0.02), myostatin (−81.6±18.1pg/ml; p=0.04), and FGF21 (−9.5±1.1pg/ml; p=0.03), and the remaining myokines did not change significantly.
Biochemical parameters (mean±standard deviation).
| Baseline | 12 weeks | p | |
|---|---|---|---|
| Glucose (mg/dl) | 92.4±3.9 | 93.1±2.5 | 0.44 |
| Total cholesterol (mg/dl) | 201.1±21.2 | 185.1±12.6 | 0.36 |
| LDL-cholesterol (mg/dl) | 119.1±23.6 | 117.2±17.3 | 0.32 |
| HDL-cholesterol (mg/dl) | 57.1±12.6 | 54.1±11.7 | 0.21 |
| Triglycerides (mg/dl) | 107.4±21.2 | 109.2±19.3* | 0.01 |
| Insulin (UI/L) | 15.7±1.1 | 13.2±1.2* | 0.04 |
| HOMA-IR | 3.9±0.22 | 3.5±0.1* | 0.03 |
| CRP (mg/dl) | 4.0±1.0 | 4.1±0.9 | 0.32 |
LDL-cholesterol: low density lipoprotein; HDL: high density lipoprotein; HOMA-IR: homeostasis model assessment-insulin resistance; CRP: C-reactive protein.
Myokine levels (mean±standard deviation).
| Baseline | 12 weeks | p | |
|---|---|---|---|
| Apelin (pg/ml) | 240.84±13.6 | 174.42±10.6* | 0.04 |
| Myostatin (pg/ml) | 782.8±17.2 | 701.3±12.6 | 0.04 |
| FABP3 (pg/ml) | 1221.9±83.6 | 1084.8±77.4* | 0.03 |
| IL-6 (pg/ml) | 12.1±1.1 | 8.1±1.0* | 0.02 |
| IL-15 (pg/ml) | 2.94±0.4 | 2.44±0.3 | 0.28 |
| FGF21 (pg/ml) | 71.9±12.5 | 62.4±9.3* | 0.03 |
| Irisin (pg/ml) | 351.4±11.3 | 338.4±8.9 | 0.54 |
| LIF (pg/ml) | 30.9±6.2 | 21.5±3.9 | 0.78 |
| Osteonectin (ng/ml) | 105.5±21.2 | 100.7±11.9 | 0.33 |
| Musclin (ng/ml) | 80.2±10.2 | 75.4±9.9 | 0.49 |
FABP3: fatty acid binding protein-3; FGF21: fibroblast growth factor 21; IL-6: interleukin-6; IL-15: interleukin 15; LIF: leukaemia inhibitory factor.
Finally, the patients were classified according to the presence of Metabolic Syndrome (MS), 32.0% of the patients presenting with MS before starting the physical exercise programme with 16% hypertension, 20% hyperlipidaemia, 8% glucose metabolism disorder and 4% sleep apnoea syndrome. After completing the programme, the MS percentage decreased to 12.0% (p=0.02), 8% hypertension, 8% hyperlipidaemia, 4% glucose metabolism disorder and 4% sleep apnoea syndrome. Before starting the physical exercise programme, 100% of the patients had at least one ATPIII MS criteria and 36% had 2 or more MS criteria. After 12 weeks of physical exercise, only 92% of the patients presented one or more criteria for MS and 24% two or more criteria (p=0.03), with 8% of the patients without any criteria for MS.
DiscussionIn our study, the prescription of physical exercise with an online platform for 12 weeks to sedentary females with obesity deceased body weight and fat mass and increased muscle mass. On the other hand, insulin resistance and the presence of metabolic syndrome improved, with a significant decrease in serum levels of apelin, myostatin, FABP-3, IL-6, and BDNF.
Intervention with physical exercise through online platforms can be an interesting methodology to reduce a sedentary lifestyle and improve the health of females with obesity. Some works have already shown the good acceptance of this type of tool in people with obesity.9–10 Moreover, its effect on anthropometric and myokine levels have not been evaluated at the same time to date. This lack of studies is secondary to multiple factors; difficulty of designing online tools, the low adherence of patients with obesity to exercise and the difficulty of designing randomised clinical trials with these tools to assess their efficacy.
The body weight loss and body mass index improvement (BMI) observed in our study is similar to that reported in other studies with face-to-face physical exercise interventions8 and very similar to other studies that combined face-to-face physical exercise interventions with controlled caloric intake.11 Previous studies have also shown a decrease in other adiposity parameters with physical exercise, such as waist circumference and fat mass12 and associated comorbidities such as hypertension.13
In the other hand, the metabolic improvements observed in our group with a physical exercise programme through an online platform are similar to those found in the literature with face-to-face physical exercise prescription.14 For example, the decrease in insulin resistance in patients with obesity, together with the decrease in the number of criteria that are part of the metabolic syndrome, is a relevant finding. In a study carried out with sedentary pre-diabetic women with obesity with a duration of 12 weeks, post-intervention blood glucose levels decreased significantly.15 In another study with an 8-week programme with physical exercise sessions, they significantly decreased insulin resistance in subjects with hyperglycaemia and hyperlipidaemia or patients’ glucose intolerance.16
In addition to all above-mentioned benefits secondary to body weight and fat mass loss, it is necessary to highlight the increase in muscle mass observed in our study. Sánchez et al.17 in a physical exercise intervention study with 10 patients with obesity undergoing bariatric surgery, demonstrated an increase in lean mass after 2 months by impedance measurement. In our case, the increase in lean mass was detected not only by impedance, but also ultrasound of the rectus femoris quadriceps. This increase in muscle mass will surely improve the functional capacity of these patients and will improve the existing sarcopenia in many patients with obesity.3,5 As we have been able to demonstrate in this work, the performance of physical exercise in sedentary females with obesity produces anthropometric and biochemical improvements. Therefore, determining myokines’ response to physical exercise would contribute to understanding how exercise-induced myokines help to improve metabolism. Myokines are the main candidates that may enable muscle-adipose tissue cross-talk communication and improve low-grade system inflammation in subjects with obesity. Perez- Lopez et al.18 demonstrated in females, that serum Il-6 and IL15 decrease after training with two different training programmes, one of them (endurance training) and another one (concurrent training, as our protocol) for 12-weeks. Endurance training was a more effective type of exercise than a concurrent one in postmenopausal females and they had a similar effect in premenopausal females. In this study,18 serum FGF21 was only reduced in the endurance training. Muscle contraction is the main stimuli to generate muscle expression and secretion of myokines. In our present study, 12-week concurrent training with an online platform caused decreases in the circulating levels of five myokines (apelin, myostatin, FABP3, Il-6 and FGF-21). Although to date no studies have investigated this myokine response to an online training programme, these results are consistent with previous in face-to-face programmes with overweight subjects and subjects with obesity.19 In some studies, contradictory changes in myokine levels have been observed. These contradictory results can be explained by multiple factors, for example when resistance exercises predominate over endurance exercises, they may be more effective in modifying circulating myokine levels. In addition to the type and distribution of physical exercise, the following can be implied in these different changes in levels of myokines; firstly, in some studies the analytical extraction is carried out just after physical activity detecting an acute release and in others the analytical extraction is unrelated to exercise time20 and secondly, the duration of the prescribed physical activity treatment, programmes of 1 week21 vs. 1-year programmes.22
In the literature we can observe a lot of contradictory results in different myokines. For example, FGF21 acts as adipokine and myokine, and the response to exercise is inconsistent; some studies have reported an increase23 and a decrease,24 too. Irisin is another example. It has been primarily reported in skeletal muscle and adipocytes, and this myokine alters the colour of white adipocytes to brown. In the literature different results have been reported; no changes after 12 weeks of endurance training25 and increase after aerobic exercise.26,27 Myostatin is a negative regulator of muscle mass, augmenting muscle catabolism and impairing muscle synthesis.17 FABP3 may act as a myokine, matching whole-body metabolism to muscular energy demands.28 In our work there is a decrease in levels of the above-mentioned myokine together with an increase in muscle mass. Moreover, apelin as an exercise-induced factor has an anti-sarcopenic obesity function by targeting satellite cells and fat cells,29 but in our study an unexplained drop was found. Since myokines respond to exercise and are related to muscle mass increase, poor results may be attributed to the concurrent training programme.
Within the limitations of the study, we can highlight the small sample size, as well as the limited duration of the intervention (12-weeks). With a larger number of patients, the effect of different types of exercises, and of different muscle groups on myokine levels could have been evaluated. Another limitation is the absence of a control group; we compared each patient with herself at baseline. Finally, another limitation is that we only determined muscle mass but did not determine functionality with dynamometry or the chair test. Despite these limitations these deficiencies are offset by strengths such as the use of an online exercise prescription platform in a real-world study, which allowed the patient with obesity to perform physical exercises in their usual environment. During the COVID-19 pandemic, home-based programmes supported by digital solutions are commonly used to maintain an adequate level of physical activity. But in the digital age in which we are immersed, simple mobile applications have managed to demonstrate an increase in adherence to physical exercise.30
In conclusion, the prescription of physical exercise with an online platform for patients with obesity modifies levels of myokines with a significant decrease in serum levels of apelin, myostatin, FABP-3, IL-6, and BDNF. Besides, a decrease in body weight and body fat mass, an increase in muscle mass, with a decrease in insulin resistance were detected. This tool is a low-cost method that facilitates the work of prescribing physical exercise. This allows the patient to have a pattern of physical exercise adapted to her individual characteristics. Future lines of research should be considered, evaluating longer periods of exercise training, different types of training, different populations and their application in treatment as a non-pharmacological strategy for other types of patients where physical exercise is a part of treatment and myokines could play an unknown key role. The determination of myokines in obese patients together with classic markers such as insulin resistance, could help us to carry out a more personalised prescription of physical and nutritional activity.
Authors’ contributionsDaniel Antonio de Luis designed the study and wrote the article.
Juan Jose Lopez Gomez and Olatz Izaola, conducted nutritional evaluation.
D. Primo and D. de Luis conducted biochemical evaluation.
Statement of ethicsThis study protocol was reviewed and approved by [HCVUA Committee], approval number [PI20/2062]. Written informed consent was obtained from all individual participants included in the study.
Consent to participate statementSigned informed consent was obtained in all participants. This research complies with the guidelines for human studies in accordance with the World Medical Association Declaration of Helsinki.
Data availability statementAll data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Funding sourcesNo funding.
Conflict of interestThe authors have no conflicts of interest to declare.