The aim of this study was to assess the risk factors associated to recurrent diabetic foot ulcers after implementing a new preventive comprehensive foot care (CFC) program carried out by a podiatrist and an endocrinologist at a multidisciplinary diabetic foot unit (MDFU) and its potential impact in decreasing recurrent ulcers.
Material and methodsA retrospective cohort study including consecutive patients who attended the MDFU for the first time from 2008 to 2014 complaining of a diabetic foot ulcer that finally healed. Patients were monitored until ulcer recurred or up to June 30, 2016. Maximum follow-up time was 8.1 years. Cumulative incidence of recurrent ulcers was analyzed during two periods: 2008–2010 (before CFC was implemented) and 2011–2014 (after implementation of CFC).
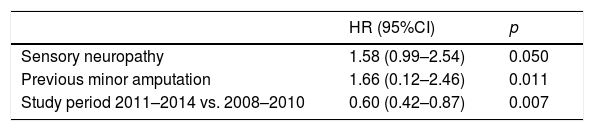
ResultsA total of 280 subjects with a median age of 69.5 years (Q25:60.2–Q75:78) were included. Of these, 64.6% were males and 92.1% had type 2 diabetes mellitus. One hundred and twenty-six (45%) suffered recurrent ulcers. Median time to recurrent ulceration was 0.97 (Q25:0.44–Q75:1.74) years. Multivariate analysis showed sensory neuropathy (HR [95% CI] 1.58 [0.99–2.54], p=0.050); minor amputation (HR [95% CI] 1.66 [0.12–2, 46], p=0.011); and 2011–2014 period versus 2008–10 period (HR [95% CI] 0.60 [0.42–0.87], p=0.007) to be factors independently associated to recurrent ulcers.
ConclusionsSensory neuropathy, minor amputation, and implementation of the CFC program were predictors of reulceration. Implementation of the CFC program was associated to a 40% reduction in reulceration. Prevention of recurrent ulcers is feasible and should be a priority in a MDFU.
El objetivo de este estudio fue evaluar qué factores de riesgo se asociaban con la reulceración en el pie diabético después de la implementación de un nuevo programa preventivo de cuidado integrado del pie (CIP), desarrollado por un podólogo y un endocrinólogo en una unidad multidisciplinar de pie diabético y su impacto potencial en reducir la tasa de reulceración.
Material y métodosEstudio de cohortes y retrospectivo que incluyó de manera consecutiva a pacientes que consultaron por primera vez por una úlcera de pie diabético durante el período 2008-2014, y que se resolvió mediante cicatrización. Los sujetos fueron seguidos hasta la reulceración o en su defecto hasta el 30 de junio de 2016, con un máximo de 8,1 años. Se analizó la incidencia acumulada de reulceraciones durante el período 2008-2010 (antes del CIP) y 2011-2014 (tras la implementación del CIP).
ResultadosSe incluyeron 280 sujetos, mediana de edad 69,5 años (P25: 60,2-P75:78); 64,6% varones y 92,1% tenían diabetes tipo 2. Ciento veintiséis (45%) se reulceraron. La mediana hasta la reulceración fue de 0,97 (P25:0,44-P75:1,74) años. El análisis multivariante demostró que la neuropatía sensitiva (HR [IC 95%] 1,58 [0,99-2,54] p=0,050); amputación menor (HR [IC 95%] 1,66 [0,12-2,46] p=0,011); y período 2011-2014 versus 2008-2010 (HR [IC 95%] 0,60 [0,42-0,87] p=0,007) se asociaron independientemente a la reulceración.
ConclusionesLos factores predictivos para reulceración fueron neuropatía sensitiva, amputación menor y la implementación del programa de CIP. La implementación del CIP se asoció con una reducción del 40% en la reulceración. La prevención de la reulceración es factible y debiera ser prioritaria en una unidad multidisciplinar de pie diabético.
Complications affecting the lower extremities of people with diabetes mellitus (DM) are a consequence of diabetic neuropathy and peripheral arterial disease (PAD), and constitute common, complex and costly problems.1 Ulceration is the most common of these complications. It is estimated that up to 25% of all patients with DM will develop ulceration during their lifetime, that 6.3% have an active ulcer, and that ulceration precedes lower limb amputation (LLA) in up to 80% of all cases.2,3 The association of these latter two events and the frequent severity of the lesions in these patients possibly account for the fact that many of our efforts are currently aimed at controlling patients with acute diabetic foot (DF).
Unfortunately, after healing, patients often suffer recurrent ulceration. In this regard, prior ulceration in itself constitutes the main predictor of recurrent ulceration.4 It is estimated that approximately 40% of all patients suffer recurrent ulceration one year after healing, versus almost 60% at three years of follow-up, and 65% at 5 years,5 with the percentage increasing even further over subsequent patient follow-up.6 This lifelong increased risk of reulceration among patients who have had a prior lesion remains an unresolved challenge in DF. As a result, some authors prefer to speak of remission, and thus of lesion-free time, rather than of cure in DF.5
The new guidelines of the International Working Group on the Diabetic Foot (IWGDF) emphasize that prevention in high-risk patients (defined as those who have experienced an ulcer or previous LLA) should receive at least the same attention as the control of acute DF.7 However, this recommendation is not regularly complied with, nor indeed is it even widely known.5
The IWGDF recently reviewed the studies analyzing interventions in reulceration in these patients, suggesting that up to 75–80% of all recurrent ulcers could be avoided if optimal use were made of all the available scientific evidence, with the integration of different interventions.8 Such interventions were grouped as follows: (1) comprehensive foot care (CFC) involving the intervention of different disciplines on multiple occasions; (2) patient self-control for monitoring skin temperature; (3) patient education; (4) therapeutic footwear; and (5) preventative foot surgery.9 In relation to these interventions, the IWGDF emphasizes the importance of including the podiatrist in the different levels of DF care.7,9
During 2008, a DF clinic was opened, attended by an endocrinologist and a podiatrist, with the aim of providing care for patients with DF at Hospital Universitario Príncipe de Asturias (HUPA) (Madrid, Spain). The coordination of different disciplines was gradually established, giving rise to a Multidisciplinary Diabetic Foot Unit (MDFU) involving different specialties: vascular surgery, general surgery, vascular and interventional radiology, traumatology, infectious diseases, and physical medicine and rehabilitation.10 Starting in 2011, a new service in the form of a CFC program was offered to patients consulting due to ulceration following the resolution of a previous ulcer.
The present study was carried out to explore the predictive factors of reulceration and to determine whether the aforementioned new CFC service results in a decrease in the recurrent ulcer rate.
Material and methodsA retrospective, single-center cohort study was carried out to analyze reulceration in diabetic patients after first consultation due to DF ulceration at the MDFU of the HUPA. From 1 February 2008 to 31 December 2014 we enrolled first consulting patients in which the ulcer lesion was resolved through healing or minor amputation. Subjects with major amputation or follow-up times of less than three months were excluded.
Patients were followed-up on until reulceration, until death in the absence of reulceration, or until the last date on which data could be obtained from the electronic case history (the last date recorded being 30 June 2016).
The patients seen came from the recruitment area of the HUPA, consisting of a large urban municipality (Alcalá de Henares) and 12 nearby towns (see details in a previous article11).
Description of the multidisciplinary diabetic foot unitThe functioning of the MDFU is described in greater detail in a previous article.11 Patients with DF lesions in our healthcare area were preferentially referred to the DF clinic, following the establishing of a diagnostic and therapeutic protocol on the lesion or lesions based on the guidelines of the International Consensus on the Diabetic Foot,7 and the interventions were coordinated with other specialties as required. All patients were followed-up on in the DF clinic until the end of the episode.
Following the episode, with healing and/or amputation, all patients were discharged to their reference physicians for follow-up, with recommendations and preventive measures such as monitoring by Podiatry with its own resources and the footwear to be used. In 2011, following the expansion of the services of the MDFU, a CFC program was started to monitor all patients who subsequently consulted due to ulceration. The CFC program consisted of individualized periodic controls every 1–3 months by a podiatrist and an endocrinologist, with the purpose of offering the following: (1) chiropodist foot care: nail treatment, callus removal, and the use of digital releasing devices (e.g., silicone orthoses); (2) counseling on the need for the continued use of definitive foot orthoses and therapeutic shoes; (3) an intensification of the educational aspects on the occasion of each medical follow-up visit; (4) preventive surgery to secure pressure release (fundamentally in the form of arthroplasty, toe flexor tenotomy and metatarsal head osteotomy [MHO]) in patients with recurrent ulcers in which conservative management had proved ineffective; and (5) the monitoring of metabolic control and comorbidities.
Data collection and processingThe clinical characteristics of the patients were collected from a database specifically designed for patient follow-up at the MDFU. Information regarding follow-up was also collected from the HORUS platform in order to improve the compilation of the variables and to more accurately assess current patient status. This platform allows access to the primary care electronic case history, as well as to the reports of the hospitals of the Madrid Health Service (SERMAS), and is shared by the entire Community of Madrid.
The following terms were used: healing if the patient maintained the skin intact for at least four weeks; reulceration defined as the development of a new DF ulcer following first consultation after healing; recurrence if the new ulcer appeared at the same site as the initial lesion; renal dysfunction defined as the first morning urine albumin/creatinine ratio (at least 2 measurements) >30mg/g or the estimated glomerular filtration rate (MDRD-4 formula) <60ml/min; sensory neuropathy in the absence of sensitivity with the monofilament (10g) and/or the tuning fork test (64–128Hz); ischemic injury defined as absent distal pulses or confirmed by diagnostic tests: the ankle-brachial index (ABI) <0.9 and/or the finger-brachial index (FBI) <0.6 and/or transcutaneous oxygen pressure (TcPO2) <30mmHg; severity of ulceration based on Wagner staging 1–512 and the grouped Texas classification12 (1=1A, 2A, 1B, 2B; 2=3A, 3B; 3=1C, 1D, 2C, 2D, 3C, 3D), the most severe being reported in the event of multiples; and the grading of infection according to the IWGDF/IDSA criteria (grades 0–3).13
Data reporting and statistical analysisQuantitative data were expressed as the median (P25–P75) and range, with confidence intervals [95%CI] where appropriate, while qualitative data were reported as absolute values and percentages (%).
The chi-squared test was used to compare qualitative variables, while the Mann–Whitney U-test was used for quantitative variables. Recurrent ulceration was assessed based on Kaplan–Meier survival and function analyses. The evaluation of reulceration predictor variables was carried out using univariate and multivariate Cox regression analyses adjusted to independent variables, with a backward stepwise selection of variables. The measure of risk was represented through the odds ratio (OR) (95%CI) and hazard ratio (HR) (95%CI) as required. In order to determine whether there were differences in reulceration rates after implementation of the CFC program, we contrasted the period 2008–2010 (before the start of CFC activity) versus the period 2011–2014 (i.e., following implementation of the program). We estimated the cumulative incidence of reulceration at 1.5 and 3 years of follow-up. The SPSS version 19.0 statistical package was used throughout. Statistical significance was considered for p<0.05.
Ethical considerationsThe present study was approved by the Clinical Research Ethics Committee of the HUPA (reference OE 26/2015). Since this was a retrospective observational study, patient informed consent was not requested. In some cases, patients were no longer followed-up on by the MDFU or had died before the start of the study. Patient data were anonymized to preserve confidentiality.
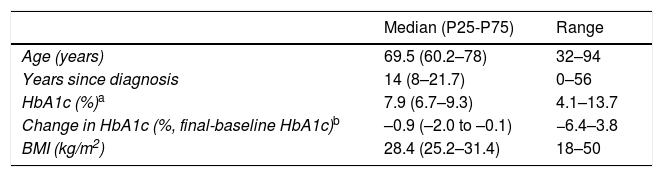
ResultsA total of 345 subjects were seen in the MDFU during the period 2008–2014. We excluded 34 patients subjected to major LLA, 18 who died with the initial lesion, and 13 subjects with a follow-up of less than three months. The final sample therefore comprised 280 subjects. Table 1 shows the most relevant data, while the characteristics of the initial ulcer leading to the first visit of the patient are reported in Table 2.
Clinical characteristics of the patients.
| Median (P25-P75) | Range | |
|---|---|---|
| Age (years) | 69.5 (60.2–78) | 32–94 |
| Years since diagnosis | 14 (8–21.7) | 0–56 |
| HbA1c (%)a | 7.9 (6.7–9.3) | 4.1–13.7 |
| Change in HbA1c (%, final-baseline HbA1c)b | –0.9 (–2.0 to –0.1) | −6.4–3.8 |
| BMI (kg/m2) | 28.4 (25.2–31.4) | 18–50 |
| n | % | |
|---|---|---|
| Type of DM | ||
| DM1 | 17 | 6.1 |
| DM2 | 258 | 92.1 |
| Secondary DM | 5 | 1.8 |
| Gender | ||
| Males | 181 | 64.6 |
| Females | 99 | 35.4 |
| Smoking | ||
| Never | 146 | 52.1 |
| Ex-smoker | 85 | 30.4 |
| Active smoker | 49 | 17.5 |
| Alcohol consumption (♀>25g/day, ♂>40g/day) | ||
| Never | 198 | 70.7 |
| Previous alcohol consumption | 46 | 16.4 |
| Current alcohol consumption | 36 | 12.9 |
| Treatment of hyperglycemia | ||
| Without drugs for hyperglycemia control | 15 | 5.4 |
| Oral antidiabetic drugs and/or non-insulin injections | 105 | 37.5 |
| Insulin±oral antidiabetic drugs and/or non-insulin injections | 160 | 57.1 |
| Retinopathy | 164 | 60.1 |
| Severe retinopathy and/or macular edema requiring treatment | 85 | 31.3 |
| Renal dysfunction (albumin/creatinine ratio >30mg/g and/or GFR<60ml/min) | 118 | 42.1 |
| Glomerular filtration rate | ||
| GFR>60ml/min | 214 | 76.4 |
| GFR 60–30ml/min | 45 | 16.1 |
| GFR<30ml/min | 9 | 3.2 |
| Dialysis | 10 | 3.6 |
| Post-transplantation | 2 | 0.7 |
| Arterial hypertension | 222 | 79.3 |
| Ischemic heart disease | 104 | 37.1 |
| Cerebrovascular disease | 40 | 14.3 |
| Ischemic heart disease and/or cerebrovascular disease | 123 | 43.9 |
| Sensory neuropathy | 214 | 76.4 |
| Previous minor amputation | 62 | 22.1 |
DM: diabetes mellitus; GFR: glomerular filtration rate; BMI: body mass index.
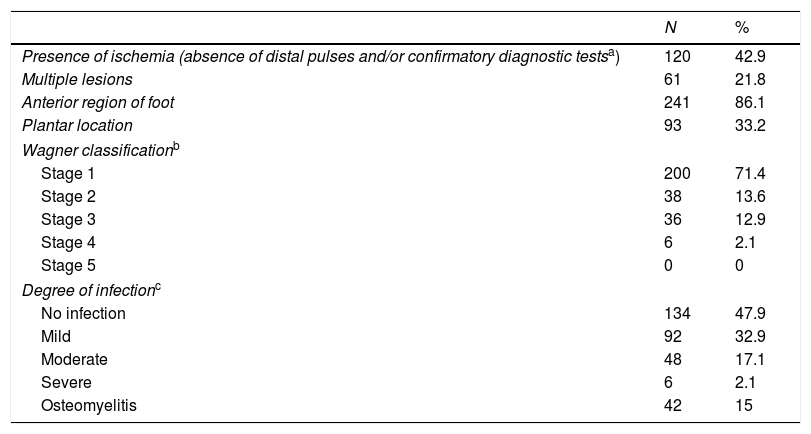
Characteristics of the first ulcer leading to consultation.
| N | % | |
|---|---|---|
| Presence of ischemia (absence of distal pulses and/or confirmatory diagnostic testsa) | 120 | 42.9 |
| Multiple lesions | 61 | 21.8 |
| Anterior region of foot | 241 | 86.1 |
| Plantar location | 93 | 33.2 |
| Wagner classificationb | ||
| Stage 1 | 200 | 71.4 |
| Stage 2 | 38 | 13.6 |
| Stage 3 | 36 | 12.9 |
| Stage 4 | 6 | 2.1 |
| Stage 5 | 0 | 0 |
| Degree of infectionc | ||
| No infection | 134 | 47.9 |
| Mild | 92 | 32.9 |
| Moderate | 48 | 17.1 |
| Severe | 6 | 2.1 |
| Osteomyelitis | 42 | 15 |
The patients were followed-up on for a maximum of 8.1 years, and those who remained free of new ulcers were followed-up on for 2.57 (1.22–4.84) years. During follow-up, 126 patients (45%) suffered a new ulcer event; 52 of these new events corresponded to ulcer recurrences (41.3%), while 74 (58.7%) represented reulceration at a site different from that of the primary ulcer.
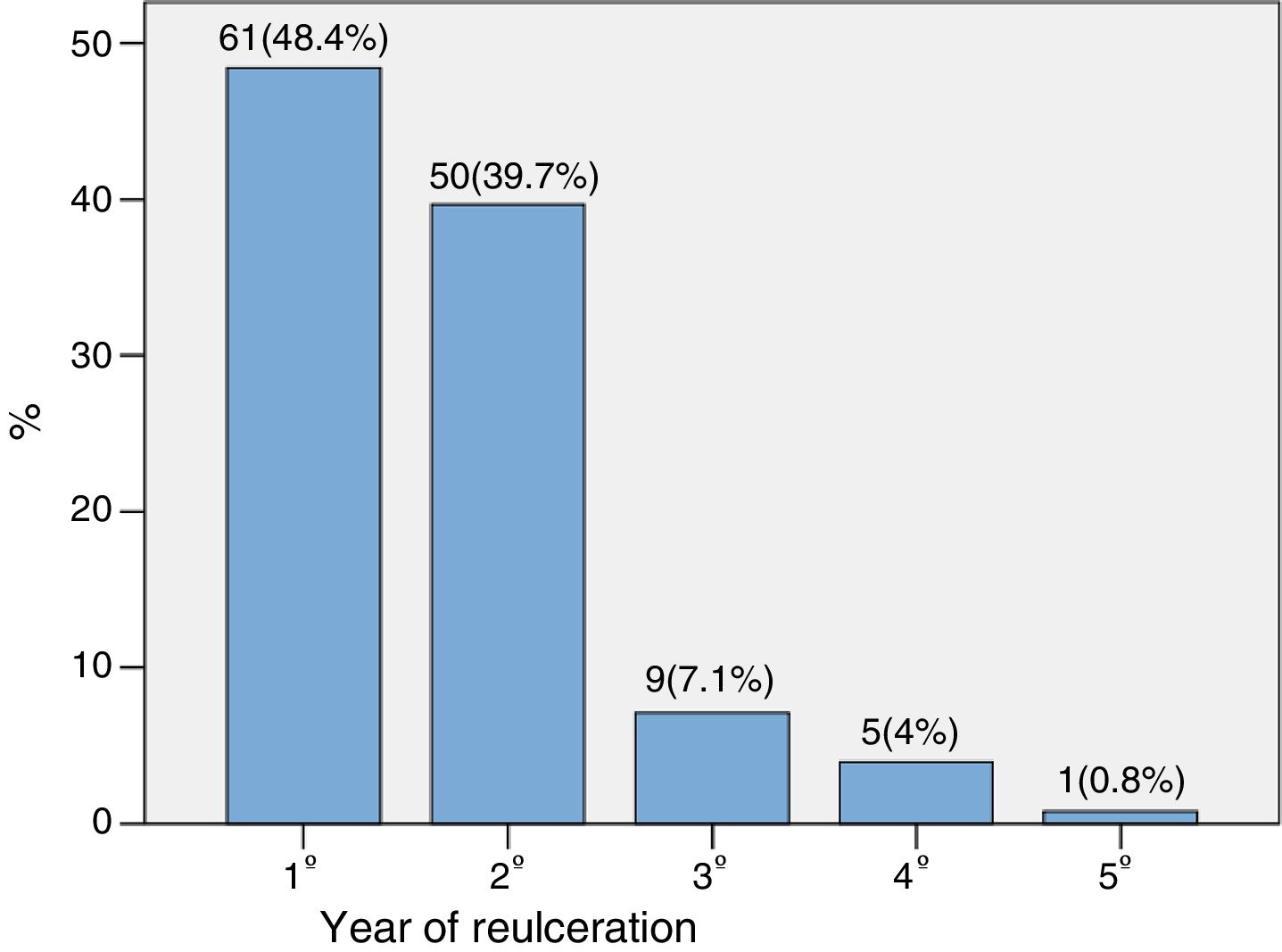
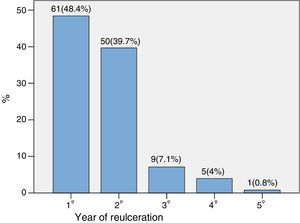
The time to reulceration was 0.97 (0.44–1.74) years. Fig. 1 shows the percentage distribution according to the year in which reulceration occurred. As can be seen, 88.1% of the new ulcers appeared in the first two years.
In terms of location, the new events were distributed as follows: 60.3% at toe level, 24.6% on the metatarsals, 6.4% at mid-foot level, and 8.7% on the heel. We examined the correlation of reulceration grouped into the anterior versus the posterior foot region with respect to the location of the initial ulcer: anterior region (84.9%) and posterior region (15.1%). Reulceration of the anterior region of the foot (91.4%) was seen to be more common than reulceration in the posterior region (8.6%) when the initial ulcer was located in the anterior region of the foot (p<0.001), with an OR of 9.70 (95%CI: 3.24–29).
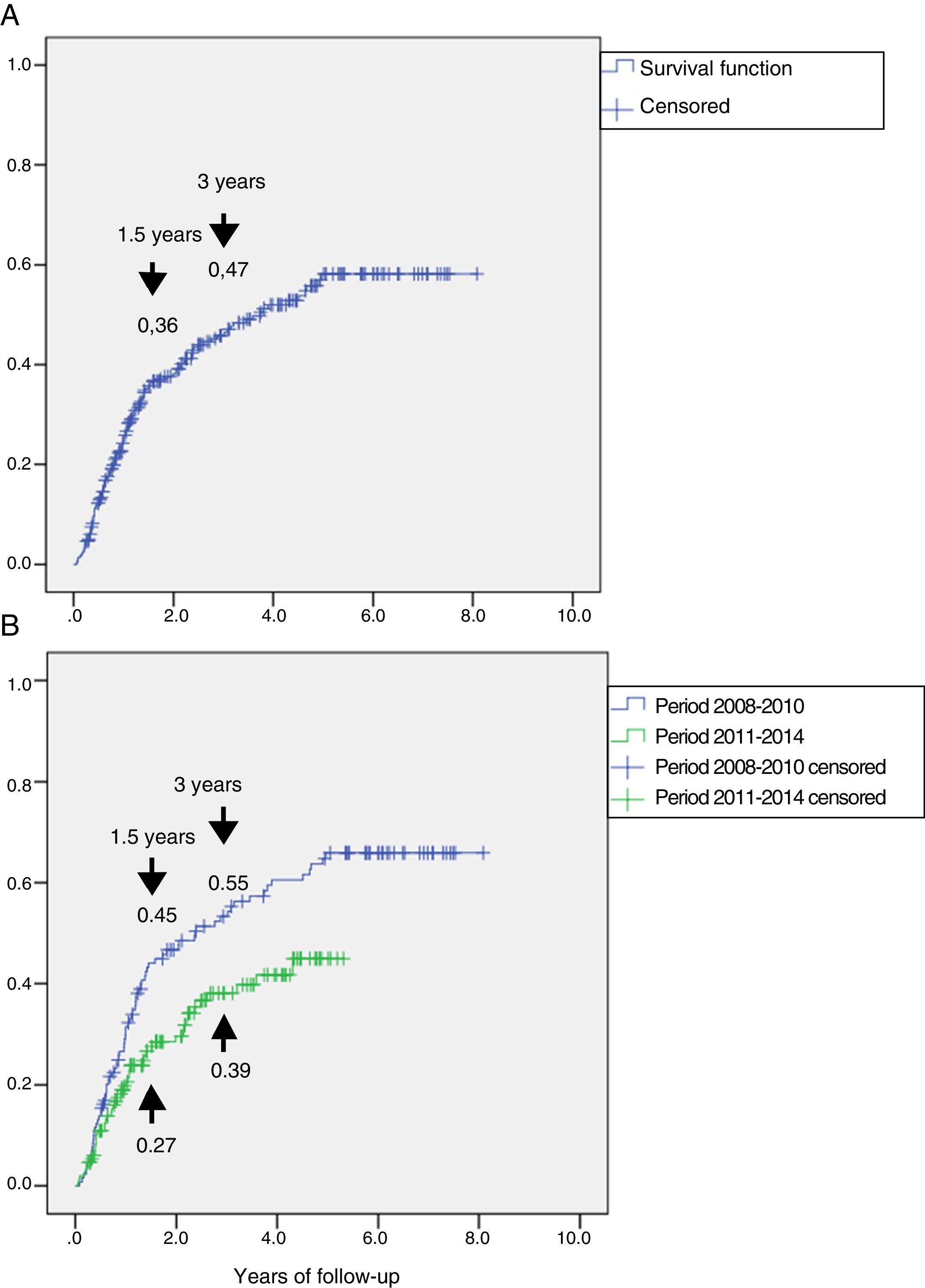
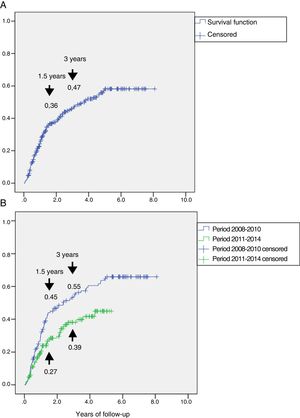
Fig. 2A shows the cumulative incidence of reulceration in the global subjects. It was estimated that 1.5 years after the first lesion, 36% of the patients presented reulceration, while at three years the percentage increased to 47%.
Representation of the cumulative incidence of reulceration (function: 1 minus survival). Panel A: cumulative incidence in the total group, which was 36% at 1.5 years and 47% at 3 years of follow-up. Panel B: cumulative incidence in the group followed-up on during the period 2008–2010 and in the group followed-up on during the period 2011–2014, showing a reduction from 45% to 27% at 1.5 years and from 55% to 39% at 3 years of follow-up, with HR 0.60 (95%CI: 0.42–0.87) (p=0.007).
A study was made to determine whether there were differences in reulceration after implementation of the CFC program in 2011. To this effect, a sub-analysis was performed of the recurrent ulcerations in the two periods, i.e., 2008–2010, prior to implementation of the CFC program (n=130) and 2011–2014, after implementation of the program (n=150). In the period 2008–2010, 77 patients suffered reulceration (59.2%) versus 49 patients (32.7%) in the period 2011–2014. The survival analysis is shown in Fig. 2B. The incidence of reulceration was seen to be lower in the period 2011–2014 than in 2008–2010, with HR 0.60 (95%CI: 0.42–0.87) (p=0.007).
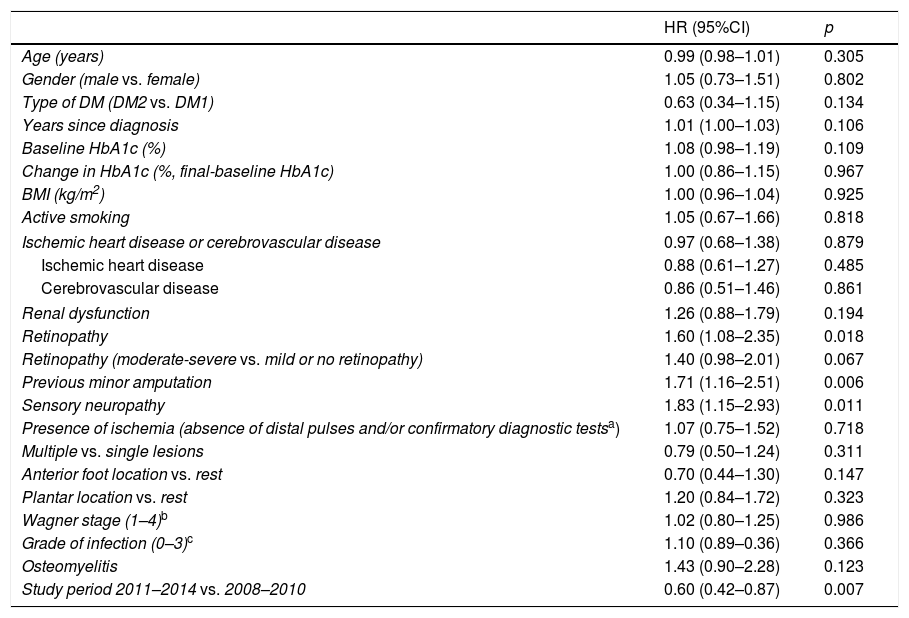
Table 3 shows the univariate Cox regression analysis of the variables predicting reulceration. The baseline variables found to be significant predictors of reulceration in the univariate analysis were entered in the multivariate analysis (Table 4). Sensory neuropathy (p=0.05), previous minor amputation (p=0.011) and the study period 2011–14 versus 2008–2010 (p=0.007) were found to be independent predictors of reulceration.
Predictors of reulceration. Univariate analysis.
| HR (95%CI) | p | |
|---|---|---|
| Age (years) | 0.99 (0.98–1.01) | 0.305 |
| Gender (male vs. female) | 1.05 (0.73–1.51) | 0.802 |
| Type of DM (DM2 vs. DM1) | 0.63 (0.34–1.15) | 0.134 |
| Years since diagnosis | 1.01 (1.00–1.03) | 0.106 |
| Baseline HbA1c (%) | 1.08 (0.98–1.19) | 0.109 |
| Change in HbA1c (%, final-baseline HbA1c) | 1.00 (0.86–1.15) | 0.967 |
| BMI (kg/m2) | 1.00 (0.96–1.04) | 0.925 |
| Active smoking | 1.05 (0.67–1.66) | 0.818 |
| Ischemic heart disease or cerebrovascular disease | 0.97 (0.68–1.38) | 0.879 |
| Ischemic heart disease | 0.88 (0.61–1.27) | 0.485 |
| Cerebrovascular disease | 0.86 (0.51–1.46) | 0.861 |
| Renal dysfunction | 1.26 (0.88–1.79) | 0.194 |
| Retinopathy | 1.60 (1.08–2.35) | 0.018 |
| Retinopathy (moderate-severe vs. mild or no retinopathy) | 1.40 (0.98–2.01) | 0.067 |
| Previous minor amputation | 1.71 (1.16–2.51) | 0.006 |
| Sensory neuropathy | 1.83 (1.15–2.93) | 0.011 |
| Presence of ischemia (absence of distal pulses and/or confirmatory diagnostic testsa) | 1.07 (0.75–1.52) | 0.718 |
| Multiple vs. single lesions | 0.79 (0.50–1.24) | 0.311 |
| Anterior foot location vs. rest | 0.70 (0.44–1.30) | 0.147 |
| Plantar location vs. rest | 1.20 (0.84–1.72) | 0.323 |
| Wagner stage (1–4)b | 1.02 (0.80–1.25) | 0.986 |
| Grade of infection (0–3)c | 1.10 (0.89–0.36) | 0.366 |
| Osteomyelitis | 1.43 (0.90–2.28) | 0.123 |
| Study period 2011–2014 vs. 2008–2010 | 0.60 (0.42–0.87) | 0.007 |
DM: diabetes mellitus; HR: hazard ratio; BMI: body mass index.
Predictors of reulceration. Multivariate analysis.a
| HR (95%CI) | p | |
|---|---|---|
| Sensory neuropathy | 1.58 (0.99–2.54) | 0.050 |
| Previous minor amputation | 1.66 (0.12–2.46) | 0.011 |
| Study period 2011–2014 vs. 2008–2010 | 0.60 (0.42–0.87) | 0.007 |
Recurrent ulceration in patients with DF after healing of a previous ulcer is a common event,5 with figures that have not changed in recent years.14,15 As such they constitute an unresolved problem. The present study confirms the magnitude of the problem, though the implementation of a CFC program coordinated by a podiatrist and an endocrinologist, within the context of a multidisciplinary team, was found to be associated with a 40% decrease in the reulceration rate among patients with DF.
In our series, after resolution of the initial ulcer (complete healing and/or minor LLA), 45% of the subjects suffered a new ulcer event, mainly during the first two years of follow-up. A recent review published by Armstrong et al.5 compiled a list of 19 studies identified in PubMed (10 observational studies and 9 randomized controlled trials) and analyzed the incidence of recurrent ulcerations in them. The review estimated that globally 60% of the subjects suffered reulceration at three years of follow-up, with no major changes at 5 years.5 These data are consistent with the findings of our own sample, in which the reulceration rate was seen to be 47% after three years of follow-up. Our follow-up time of as long as 8.1 years, with 75% of the subjects free from reulceration when monitored up to 4.84 years, exceeds the mean duration of follow-up of the studies cited in the review. Indeed, it reported a maximum follow-up of three years in 15 of the 19 studies, and most of them did not exceed 18 months.5
A less extensively studied aspect is the correlation of reulceration to the location of the first ulcer. In the present study, reulceration was more commonly observed in the anterior region of the foot when the previous ulcer was likewise located in that region. Furthermore, 41.3% of the new ulcers appeared in the same location as the first lesion and therefore constituted recurrences. These data agree with those reported by Peters et al., who found 42% of the reulcerations to appear in the same location when the initial lesion was located on the hallux or metatarsal heads.16
These data have important practical implications, since reulceration commonly occurs in the same location, and preventive efforts therefore should be intensified in the case of lesions in the anterior region of the foot. The measures should comprise both footwear therapy and orthopodiatric treatments, since the anterior region is more vulnerable to reulceration. Preferential ulceration in the region of the toes, with a prevalence of 60.3% in our series, is related to the profile of the patients seen in the MDFU. In effect, practically 50% of all the cases seen in the Unit correspond to ischemic lesions.11 This coincides with the findings of other series with profiles similar to our own,16 but differs from the data reported by a center participating in the Eurodiale study, where peripheral arterial disease was scantly prevalent and lesions of the sole of the foot were seen to predominate.15
The univariate analysis allowed us to explore the contribution of each of the baseline variables to the risk of reulceration. The presence of diabetic retinopathy, prior minor amputation, sensory neuropathy, and the study period involved were found to be predictors of reulceration, though only the last three parameters persisted as independent risk factors in the multivariate analysis.
Few studies have examined which risk factors are independently associated with reulceration.15–20 In those studies that are available, research proves to have been partial, since not all the variables with a potential role are taken into consideration in them. Furthermore, the studies are heterogeneous, since they include cases with different etiopathogenic backgrounds (a different prevalence of peripheral arterial disease or only neuropathic cases). In turn, some only include patients that have suffered reulceration in very specific locations such as at plantar level. Lastly, some of the studies also evaluate interventions with therapeutic footwear.19 This leads to significant bias, with almost no agreement among the reported results.
The increased risk of reulceration detected in our study in subjects with retinopathy is not surprising, considering that the underlying etiopathogenesis coincides with that of neuropathy, and that it is more common and more severe in patients with DF ulcer.20 However, the few studies on this issue have found no correlation to the risk of reulceration.16,17 The study published by Winkley et al. found microvascular complications (combined retinopathy, nephropathy and diabetic neuropathy) to be independently associated with an increased risk of reulceration.21
The presence of neuropathy detected by a loss of sensitivity with the monofilament or tuning fork test was shown to be an independent risk factor for reulceration, as was reported in the IWGDF guidelines.7 This is moreover consistent with the observations of two prospective studies in patients both with concomitant peripheral arterial disease17 and with only neuropathic DF.18
Minor amputations have been analyzed in some studies as a predictor of reulceration.15–19 They have not been shown to represent a risk factor for new lesions, though their presence is included in category 3 of the IWGDF risk DF classification.7 In our series, minor amputation was the predictor with the strongest independent correlation to reulceration, with a HR of 1.66. Such increased reulceration could be explained by the transfer of plantar pressures to areas close to the initial lesion, where resection of one or more metatarsal heads was carried out.22 Accordingly, in these patients we should intensify preventive care, releasing pressure particularly in the anterior region of the foot.
No association was found between reulceration and peripheral arterial disease or the degree of blood glucose control. However, in the reviewed series, only one established an association with peripheral arterial disease16 and another with glycosylated hemoglobin (HbA1c).15 The decrease in HbA1c was likewise not associated with a lesser risk of reulceration, despite the fact that more intensive control has been associated with a lesser risk of amputations in a recent meta-analysis.23 Little therefore can be stated as to whether blood glucose control plays a role in the risk of reulceration. One aspect that should be taken into account is that better control implies not only an improved blood glucose environment, but is usually also associated with better adherence to treatment recommendations in general.
The most prominent aspect of this study was the analysis of the cumulative incidence of reulcerations in patients followed-up on in two different time periods: 2008–2010, when patients after resolution of the initial lesion were not followed-up on in the DF clinic; and 2011–2014, when patients entered the CFC program after resolution of the initial lesion. We believe the estimated 40% reduction in the incidence of reulceration during the follow-up period to be directly related to implementation of the CFC program, and therefore to be a consequence of that program.
From 2011 onwards, the MDFU remained without changes with respect to the previous period 2008–2011. The subjects assessed in both periods had very similar clinical characteristics and initial lesion profiles (additional material [Table 1]). The decreased incidence of reulceration seen in both periods therefore cannot be attributed to lesser patient complexity or substantial changes in the background disease.
The CFC program afforded preventive measures such as chiropodist foot care; intensified educational measures; provided orthoses allowing temporary interdigital pressure release; counseled patients on the need to use orthotic insoles and on their footwear (which should be of a therapeutic rather than standard kind); recommended preventive surgical procedures; ensured centralized control of blood glucose and comorbidities; and fundamentally conducted frequent physical presence monitoring, since the patients were seen with a periodicity of once every 1–3 months after the initial visit, conditioned to their personal needs and based on the patient risk stratification system of the IWGDF.7 In this follow-up process, the work of the podiatrist proved essential and allowed for the checking of adherence to the prescribed therapeutic interventions. It is important to note that the professional skills afforded by a podiatrist in the MDFU alleviated the shortcomings in the range of services offered by the public health system,24 since the inclusion of this professional in the Unit was the element that allowed for close patient follow-up and a periodicity of visits conditioned to patient risk.7
The IWGDF, through its guidelines, clearly establishes that the prevention of new ulcers is feasible in high-risk patients such as ulcerated subjects.8 A review carried out by this group showed that reulceration can be reduced as a result of different interventions in high-risk patients in 30–60% of the cases, and that this figure can reach 58–98% with treatment adherence.7
A series of interventions were grouped under the term CFC to define when one or more professionals intervened in patients on multiple occasions and with various procedures. Three interventional studies lasting two years were identified. These were basically individualized educational interventions with the offering of chiropodist care,25,26 and the third study involved group educational sessions and the offering of therapeutic shoes.27 The three studies globally showed a 30% decrease in reulceration, and this reduction was estimated to potentially reach 76% in the presence of good adherence to therapy.28,29 Our results, i.e., a 40% decrease in reulcerations, agree with the published data.
Interventions to reduce reulceration have two major objectives: (1) to reduce biomechanical factors causing mechanical stress and ulceration in a vulnerable foot; and (2) to improve patient education.30 Education is perhaps the best available resource for improving treatment adherence, which represents a genuine challenge for maintaining remission in DF patients. Combining these preventive measures with adequate control of acute DF in the context of an MDFU would lessen all DF problems in general7 and the need for LLA in the diabetic population in particular.31
The present study has a number of limitations:
- -
Some biomechanical variables that could be associated with reulceration, such as the presence of deformities, joint mobility, and gait registries, were not analyzed.
- -
The precise location and type of foot surgery prior to the new ulcer event was not recorded, except when minor amputation was performed. As a result, these parameters could not be correlated to the appearance of recurrence (new ulceration in the same location) or to reulceration in a location different from that of the primary ulcer.
The study also has a number of strong points:
- -
It was carried out in a real life setting, thus allowing the conclusions to be extrapolated to routine clinical practice.
- -
There were no losses to follow-up. All the patients were monitored in the MDFU, and supplementary data were drawn from the electronic case history through the HORUS digital platform.
- -
The follow-up period was long (up to 8.1 years), with follow-up of 75% of the subjects without reulceration for almost 5 years.
In conclusion, this study confirms that reulceration is a common problem, affecting 4–5 of every 10 diabetic patients after healing of a first ulcer. In our experience, the factors independently associated with reulceration included sensory neuropathy, a history of minor amputation, and the implementation of a preventive CFC program. Implementation of the CFC program was associated with a significant decrease in the incidence of reulcerations during follow-up. The prevention of reulceration is feasible in clinical practice and constitutes a priority measure to be considered in any MDFU.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Jiménez S, Rubio JA, Álvarez J, Lázaro-Martínez JL. Análisis de las reulceraciones en una unidad multidisciplinar de pie diabético tras la implementación de un programa de cuidado integrado del pie. Endocrinol Diabetes Nutr. 2018;65:438–438.